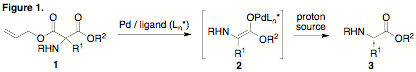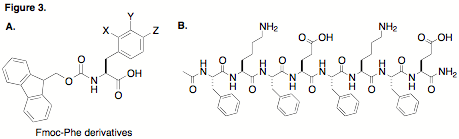www.acsprf.org
Reports: DNI148922-DNI1: Asymmetric Decarboxylative Protonation of Alpha-Aminomalonic Esters for the Synthesis of Alpha-Amino Acids
Bradley L. Nilsson, PhD , University of Rochester
a-Amino acids (a-AAs) are among the most important and useful familiesof natural products. The Nilsson group studies the self-assembly offunctionalized amino acids and peptides in order to understand thephysicochemical parameters that control self-assembly and to create novelbiomaterials. Our work has relied on the use of both canonical and noncanonicalamino acids. The research proposed in this ACS PRF project was initiated inorder to facilitate synthetic access to single enantiomers of nonnatural aminoacids in support of our studies in peptide self-assembly. The objective of ourPRF-funded research project has been to develop a novel synthetic method forthe chemical synthesis of a-AAsthat combines classical aminomalonate alkylation/decarboxylation with modernasymmetric catalysis. Specifically, we proposed the alkylation of unsymmetricalaminomalonic esters in which one of the ester substituents is an allyl group (1). The resulting compound 1 can undergo selective decarboxylation at the allylester using palladium catalysis to generate an intermediate enolate 2. We further proposed that the judicious selectionof a chiral ligand for the palladium catalyst and an appropriate proton sourcewould allow enantioselective protonation of the intermediate enolate givingrise to enantiomerically enriched a-AAs3. This strategy is operationallystraightforward with the potential for a more general substrate scope thanexisting methods.
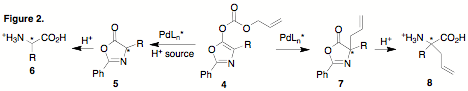
As described inthe progress report submitted following year 1 of this grant, the initialsynthetic strategy was unable to provide amino acids with highenantioselectivity. Specifically, it was observed that treatment of 1 with Pd and a variety of ligands resulted in rapiddeallylation, but that efficient decarboxylation to provide the desired aminoacid 3 was slow and requiredheating to 80 ¼C. Enantioselectivity of these reactions was poor at theseelevated temperatures. In order to promote more ready decarboxylation of theallyl ester we subsequently explored the use of electron-deficient cyclicphenyloxazol-5-one derivatives 4.It was reasoned that these derivatives have two advantages over the acyclicmalonates: 1) decarboxylation should be more facile; 2) the putative enolateintermediate is cyclic, eliminating concerns with enolate geometry.
Whiledeallylation/decarboxylation of 4 washighly efficient, other difficulties were encountered. Derivatives of 4 underwent rapid deallylation/decarboxylation and,in the absence of a proton source, reallylation at the a-carbon occurred rapidly and in highyield (>90%), providing 7. Thesecompounds were readily hydrolyzed under acidic conditions to give quaternaryamino acids 8. These quaternaryallyl amino acids are also useful products; unfortunately, enantioselectivityin the preparation of 7/8 waslow for all derivatives and catalyst ligands screened (< 8% ee).In addition, when 4 was treated with Pdin the presence of Meldrum's acid as a proton source, very little of compound 5 was isolated. Instead, it was found thatreallylation to 8 was highlycompetitive with protonation. All efforts to circumvent this competitivepathway were unsuccessful. While efforts to improve this chemistry continue andwill be reported in due course, the funds from this ACS PRF grant have alsobeen used to support elements of our work in the study of self-assembling aminoacids and peptides.
As shown inFigure 3, we have studied the self-assembly of Fmoc-Phe derivatives (Figure 3A)and amphipathic peptides (Figure 3B) in water. We have successfully shown thatFmoc-Phe derivatives undergo efficient self-assembly to form amyloid-likefibrils and that these fibrils form higher order networks with emergentproperties. Specifically, these self-assembled fibrils form functionalhydrogels. We have shown that halogenation of the Phe side chain exerts aprofound influence on self-assembly and hydrogel rates and on the resultingmechanical properties. We have also demonstrated that changes to thehydrophobic aromatic residues of amphipathic peptides significantly impacts theself-assembly properties of these peptides as well. In addition to thesefundamental studies, funding from the ACS-PRF program has been used topartially support the development of stimulus-responsive self-assemblingamphipathic peptides that only form fibrils in reducing environments.
Funding fromthe Petroleum Research Fund has had a significant impact on the early work ofthe Nilsson group. Our work is mainly focused on the study of peptideself-assembly processes. We routinely use nonnatural amino acids as probes toperturb these assembly processes and the development of novel and efficientmethods to access amino acids synthetically will be of great value to ouroverall research efforts. The PRF has enabled us to undertake this work, whichrepresents a new research direction for our group and promises to make a majorimpact on our program. The grant has supported the work of no fewer than fourgraduate students and summer research by an undergraduate student. The granthas supported work that has resulted in five publications in peer-reviewedjournals to date and work supported in two published conference proceedings.Funding from the ACS PRF program was also used to partially support travel bythe PI to several research conferences for the dissemination of our researchresults. We gratefully acknowledge the support of the donors to the ACS PRF forthe profound impact this funding has had on our research and education effortsat the University of Rochester.