www.acsprf.org
Reports: UR449939-UR4: Demystifying Microwave Assistance in Homogeneous Catalysis: Chemical Kinetics of Microwave-Heated Palladium Reactions
Andrew W. Holland, PhD , Idaho State University
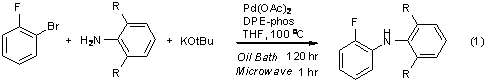
Many palladium catalyzed reactions reportedly enjoy dramatic acceleration when heated by microwaves rather than oil baths, even under otherwise identical temperatures and reaction conditions. The origin of this improvement in homogeneous systems is not understood, and this project aims to locate the source of these effects in the kinetics of these reactions. Our work this year focused on two reactions a Buchwald-Hartwig coupling we previously reported to be accelerated by microwave heating (eq 1) and a Sonogashira coupling reported, in two independent literature reports, to require widely different reaction times under microwave and conventional conditions (eq 2). We have sought to collect kinetic data for each of these reactions, under each set of conditions, to see how the impact of the microwave varies across a range of temperatures, solvents, catalyst loadings, and other reaction conditions. We hope to establish whether the microwave somehow increases reaction rates, improves catalyst lifetimes, shortens induction times, or otherwise modifies reaction progress.
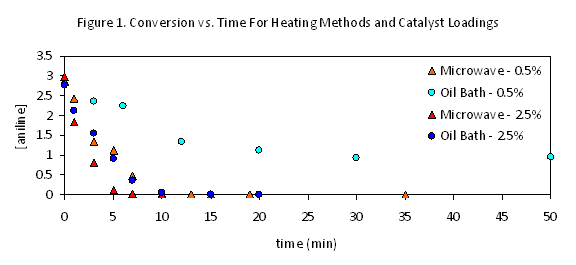
Although the Buchwald Harwig reaction illustrated in eq 1 suffers from a degree of irreproducibility depending on catalyst and solvent batches, studies of parallel reactions have repeatedly confirmed there to be significant and consistent differences between microwave and conventionally heated reactions. Conversion occurs primarily at very early reaction times with both heating methods, proceeding at roughly twice the early rate in the microwave (Figure 1). (Progress thereafter is sufficiently slow that the conventional heating must be continued for much longer, effectively exaggerating the degree of the microwave's effect.) This suggested that the duration of this initial burst of reactivity, perhaps correlating to the lifetime of the most effective catalyst generated, might be the key difference between the two reaction conditions. This behavior was not appreciably changed by moves to lower or higher temperatures. When these reactions were repeated with much higher catalyst loadings, however, the differences between microwave and conventional heating were dramatically reduced both reactions were complete within 10 minutes under these conditions, and the microwave rate was only very slightly faster. This suggests that the microwave may minimize a catalyst decomposition pathway that becomes less significant at higher loadings.
The Sonogashira reaction illustrated in eq 2 was reported to proceed 20 times as fast and in higher yield when heated in the microwave. In our hands, however, we see no dramatic difference between couplings conducted using microwave and oil-bath heating, with both methods generally yielding results similar to the shorter reaction times reported for microwave heating (Figure 2). Although the reaction is moderately sensitive to catalyst batch and reagent purity, it seems that the more likely explanation for the prior report of microwave acceleration is simply that the oil bath reaction was not monitored as frequently. In essence, the difference in reports reflects the relative patience of the researchers involved, rather than an intrinsic difference in microwave heating. We are currently beginning to explore a Suzuki coupling and a copper free Sonogashira coupling via comparisons similar to those described above.