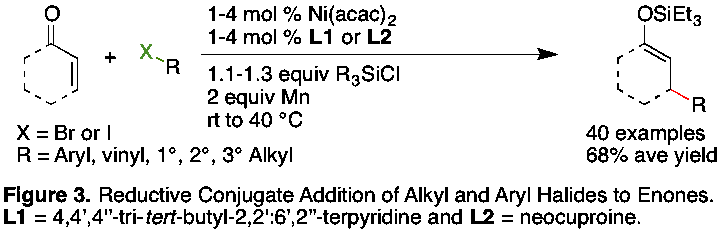www.acsprf.org
Reports: DNI148829-DNI1: Transition-Metal Catalyzed Direct Addition of Organohalides to Carbonyls and Imines
Daniel J. Weix, PhD , University of Rochester
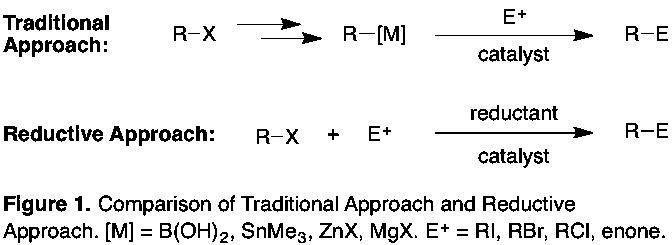
Advancements in the catalytic coupling of nucleophilic carbon reagents with carbon electrophiles has become a cornerstone of modern synthesis. The built-in bias of these substrates enables selective cross-coupling, but the need for pre-formed organometallic reagents (1) limits the number of commercially available derivatives; (2) incurs extra steps for their synthesis that are often temperature, air, and moisture sensitive; and (3) reduces the number of accessible target molecules due to functional-group compatibility and stability problems. These limitations, in turn, influence which potential drugs are synthesized and tested. Instead of synthesizing the best molecules, we often must settle for what can be easily made. Substitution of a second electrophile for the organometallic partner in cross-couplings offers the potential to dramatically increase the number and types of molecules that can be easily made because of the large number of commercially available carbon electrophiles (>1 million R-X vs. ~5 thousand R-B(OH)2) and the low cost of all components (Figure 1).
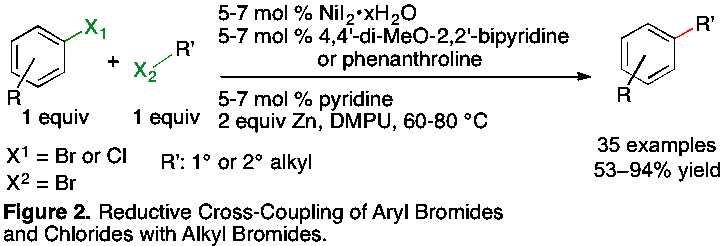
In the past year we have made significant progress towards the development general strategies for reductive alkylation of aryl bromides and activated aryl chlorides with alkyl bromides (Figure 2). Our previous work had been largely limited to aryl iodides, which are less readily available than aryl bromides or chlorides. The first examples of the alkylation of vinyl bromides were also developed. This work has also explored the functional-group compatibility of reductive coupling more extensively than any previous studies. The reaction tolerates acidic protons (N-arylsulfonamide N-H), easily hydrolyzed groups (TfO-Ar), and easily transmetalated nucleophilic carbon reagents (Me3Sn-Ar). This work has been submitted for publication.
We have also developed a reductive approach to conjugate addition that allows for the trapping of the product as a versatile silyl enol ether (Figure 3). Secondary and tertiary alkyl halides require a terpyridine ligand and aryl halides can be coupled in high yield when a hindered phenanthroline derivative, neocuproine, is part of the catalyst. Functional-group compatibility is again excellent, with acidic (Ar-NH-C(O)CF3) and electrophilic (Ar-CHO) groups well tolerated. These results were published (Org. Lett. 2011) and another manuscript has been submitted for publication.
These results have established the competiveness of reductive approaches in two key C-C bond-forming reactions, cross-coupling and conjugate addition. The major advantages of both reactions are stable, readily available starting materials and simple procedures that require no special precautions to exclude air and moisture. The Petroleum Research Fund support was instrumental for the generation of these preliminary results and the program is now funded by the National Institutes of Health.