www.acsprf.org
Reports: GB148256-GB1: Enantioselective Nucleophilic Catalysis for the Synthesis of beta-Lactams and other Nitrogen Containing Heterocycles from Isocyanates
James Arthur MacKay, PhD , Elizabethtown College
The original goals of this project were to develop a nucleophile catalyzed isocyanate-olefin cyclization to prepare β-lactams. Since the original proposal, the project has diverged in two continuing directions. The first is directly related to the original goals of isocyanate-olefin cyclizations. The second involves the exploration of an allenolate variant of the Rauhut-Currier Reaction. The recent progress on each of the projects follows.
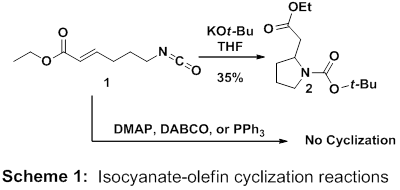
At the time of the last report, we had prepared the key cyclization precursor (1) for the intramolecular isocyanate-alkene cyclization reaction and discovered that stoichiometric base can promote cyclization. Treating 1 with potassium tert-butoxide led to the formation of pyrrolidine 2 in 35% yield (Scheme 1). Mass balances in these reactions have been problematic and possible polymerization pathways are potential culprits for low yields. In addition, we have attempted several reactions in the presence of neutral nucleophiles including DMAP, DABCO, and triphenylphospine with the ultimate goal of preparing β-lactams. Though we have evidence for the initial attack of the nucleophile into the isocyanate, to date we have still been unable to cyclize 1 using nucleophilic catalysts. This alludes to the problem of the intermediate aza-ammonium enolate being too stable for cyclization onto an unsaturated ester.
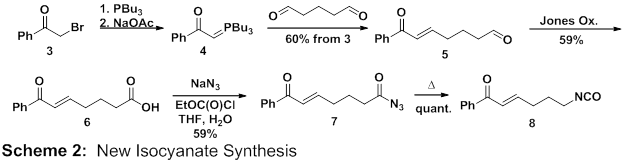
In an effort to favor the cyclization step in the key reaction, we targeted the more electrophilic substrate 8. The synthesis of 8 is a straightforward 4-pot procedure (Scheme 2). Starting from bromoacetophenone (3), phosphorane 4 is formed in situ using tributylphosphine followed by sodium acetate as a base. An aqueous solution of gluteraldehyde is then added in excess leading to a 60% yield of 5 based on 3. The major byproduct, the result of a double Wittig reaction, is easily separable using column chromatography. In our initial work, triphenylphosphine was used to make a more standard Wittig reagent. We found that the triphenylphosphine oxide byproduct is difficult to remove but tributylphosphine oxide is more easily separated by loading the crude oily product directly onto a flash column. With 5 in hand, oxidation using the Jones reagent afforded the carboxylic acid. The product formed in high yield however it has been difficult to obtain analytically pure product. Efforts to purify 6 through either acid/base extraction or silica gel chromatography afforded extensive decomposition presumably by way of intramolecular conjugate addition of the carboxylate anion. To verify this, acid 6 was stirred with dilute HCl for 24 h whereby extensive decomposition took place and NMR revealed the absence of alkene protons. As a result, acyl azide 7 was formed from unpurified 6. Chromatographic purification of 7 was required leading to a respectable 59% yield. Finally, 7 was heated to quantitatively form isocyanate 8 in a manner analogous to our synthesis of 1. This reaction sequence has been suitable for scale-up affording gram scale quantities of isocyanate 8. We also envision this general synthesis as able to afford many derivatives of 8.
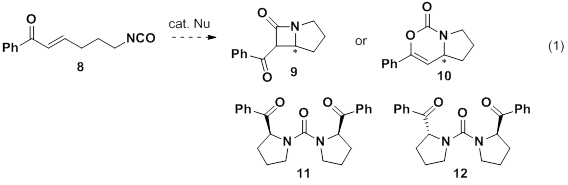
Isocyanate 8 has been treated with several nucleophiles in an attempt to induce cyclization (eq 1). Unlike, 1, this substrate appears to be more reactive in the presence of common nucleophilic catalysts including DMAP, DABCO, and Ph3P. We have yet to isolate the desired products 9 and 10, though we have recently identified meso-11 and rac-12 as products using each of the aforementioned nucleophiles. The reactions are not clean and other byproducts are yet to be identified, however the recognition of 11 and 12 as products paves the way for further optimization studies leading to chiral pyrrolidines.
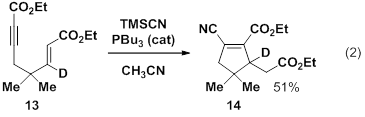
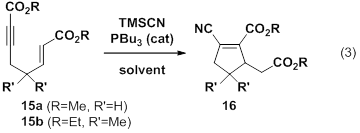
The second direction that our work is taking is in the area of an Allenolate-Variant of the Rauhut Currier reaction. As discussed in the 2010 report, we have found that trialkyphosphines catalyze the cyclization of enynes in the presence of trimethylsilyl cyanide. The net result is a chiral cyclic molecule resulting from the formation of two new carbon-carbon bonds and containing dense functionality. Though chiral catalysts lead to low selectivity, we have prepared a duterated substrate (13, eq 2) which does not appear to undergo proton transfer at the key chirality center, thus ruling out undesired racemization pathway.
In addition we have performed a solvent screen on this reaction using two substrates (Table 1). Results indicate that while acetonitrile tends to afford high yields for the cyclizations, toluene is often an acceptable (sometimes even superior) solvent as well. Dichloromethane and tetrahydrofuran gave poor yields in the two systems tested.
Entry
| Solvent
| % Yield of 16a
| % Yield of 16b
|
1
| Toluene
| 72
| 42
|
2
| DCM
| 54
| 35
|
3
| Acetonitrile
| 25
| 45
|
4
| THF
| 5
| 12
|
Table 1: Solvent Screen for the Allenolate Variant of the Rauhut-Currier Reaction
In addition to the science discussed above, this work has had a significant impact on several undergraduate chemistry students. Since the funding of this proposal, 12 undergraduate students have been trained in my research laboratory. The skills and techniques learned are invaluable toward their future endeavors as scientists. In the past year, 3 students presented their work at a The University of Maryland-Baltimore County Undergraduate Research Symposium and all three students won first place in group awards for their posters. One student presented a talk at the Intercollegiate Student Chemists Convention held this year at West Chester University. In addition, each student presents their work yearly at our local Scholarship Day in April. Five students have graduated and each has continued on to the next phase of their intended career (one to pharmacy school, two to graduate school, one to medical school and one is teaching high school chemistry). Two of those students graduated with honors in the discipline. And all 5 are members of Gamma Sigma Epsilon, the chemistry honors society.