Reports: G10
46436-G10 The Oxidation of Ethanol and Dimethyl Ether by Binary and Ternary Electrocatalysts for Fuel Cell Applications
Introduction
Proton exchange membrane fuel cells (PEMFC) hold great promise for applications such as portable power supplies and transportation. To unlock the promise of PEMFCs several challenges need to be addressed. These include sustainable fuel sources, fuel storage, cost and long term reliability. The use of ethanol in PEMFC addresses some of the problems encountered with hydrogen and methanol usage, however, the drawbacks of operating with alternative fuels, namely low activity of electrocatalysts, anode poisoning and fuel crossover has to be addressed.
In this project, new combinations of elements were explored to increase the activity of catalysts for ethanol oxidation. Platinum based ternary and quaternary component electrocatalysts were investigated.
Experimental Method
Electrocatalysts were prepared by the modified Pechini-Adams method. Metal resins were prepared by first mixing citric acid (CA) with ethylene glycol (EG) at about 60oC. Appropriate amounts of metal salts (M) were dissolved in isopropanol and then added slowly to the CA/EG mixture to obtain a M: CA: EG molar ratio of 4:16:1. The mixture was then heated at about 90oC to evaporation until about half of the initial volume was obtained.
Powdered carbon (Vulcan XC 72R) pretreated in nitrogen atmosphere (400oC for 1 hr) was added on a weight basis to the metal resin mixture aiming at a final catalyst loading of 40 wt% catalyst. The mixture was sonicated for 10 minutes and then heated through three temperature regimes in a muffle furnace. The mixture was heated from room temperature to 250oC at 1oC/min and then held for 1 hr. The temperature was then raised to 350oC at 15oC/min and then maintained for 30 minutes; and finally to 450oC at 30oC/min and held for 2 hrs. Upon cooling, electrocatalysts were again heated to 500oC for 90 minutes to rid them of any excess carbon.
The electrocatalysts were subsequently characterized by energy dispersive X-ray analysis (EDAX), transmission electron microscope (TEM), cyclic voltammetry (CV) in 0.5M H2SO4 and 1M C2H5OH, and chronoamperometry (CA) in 0.5M H2SO4 and 1M C2H5OH.
Results and Discussion
The compositions of ternary and quaternary catalysts produced were analyzed by EDAX with some of the results presented in Table 1. The EDAX results are less conformable to the initial nominal compositions than desired. It appears that a peak overlap between Pt and Ir may cause difficulty in calculating the composition of the catalyst. It also appears that the nickel and chromium may be lost preferentially during the process. More research is needed in this area.
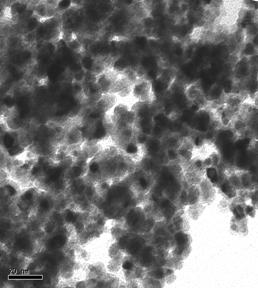
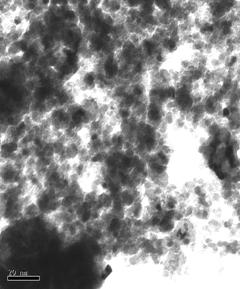
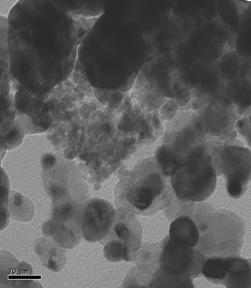
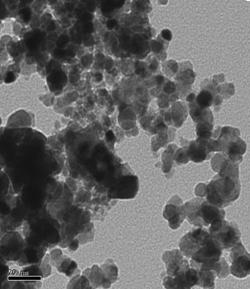
TEM images of some of the prepared catalysts are shown in Figure 1. Particle size distribution of studied electrocatalysts varied between 2-10nm, however, as it can be seen in the ternary catalysts images, agglomerates also formed varying between 20-50nm.
Table 1: EDAX analysis of Pt-based catalysts prepared by Pechini-Adams method
|
Electrocatalysts
|
Nominal Composition (Wt %)
|
Determined by EDAX (Wt %)
|
||||||||
|
Pt |
Ir |
Sn |
Cr |
Ni |
Pt |
Ir |
Sn |
Cr |
Ni |
|
|
PtIrCr/C |
70 |
15 |
- |
15 |
- |
70 |
25 |
- |
5 |
- |
|
PtIrNi/C |
70 |
15 |
- |
- |
15 |
61 |
31 |
- |
- |
8 |
|
PtIrSn/C |
70 |
15 |
15 |
- |
- |
52 |
30 |
18 |
- |
- |
|
PtIrSnCr/C |
70 |
10 |
15 |
5 |
- |
56 |
21 |
18 |
5 |
- |
|
PtIrSnCr/C |
70 |
15 |
10 |
5 |
- |
56 |
23 |
17 |
4 |
- |
|
PtIrSnCr/C |
70 |
15 |
10 |
5 |
- |
60 |
25 |
14 |
1 |
- |
|
PtIrSnCr/C |
65 |
15 |
10 |
10 |
- |
41 |
39 |
17 |
3 |
- |
|
PtIrSnNi/C |
70 |
15 |
10 |
- |
5 |
60 |
27 |
10 |
- |
3 |
|
PtIrSnNi/C |
65 |
15 |
10 |
- |
10 |
63 |
19 |
16 |
- |
2 |
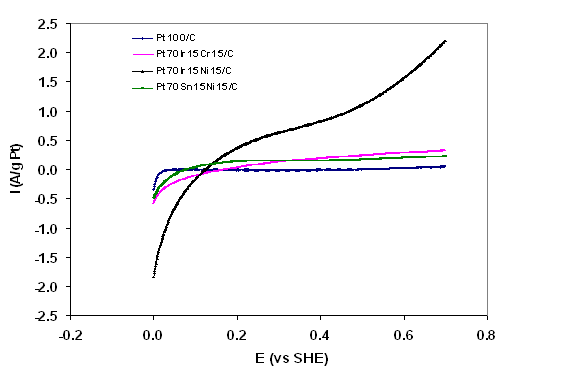
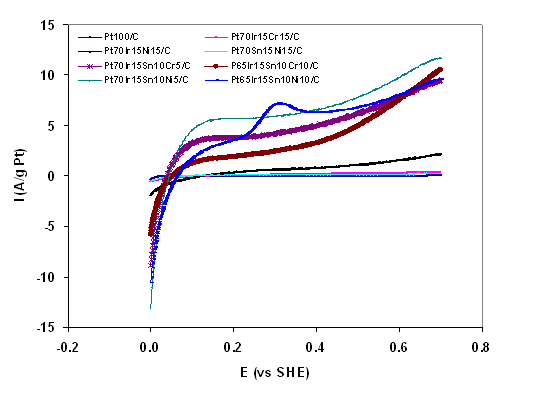
The oxidation scan produced by CV in the presence of ethanol for several electrocatalysts are depicted in Figures 2A and 2B. It is evident from Figures 2A and 2B that the ternary and quaternary catalysts increase the current produced and thus the rate of ethanol oxidation as compared to Pt100/C. The quaternary catalysts in turn exhibit greater catalytic activity for ethanol oxidation than the tertiary catalysts. The current produced at 0.7V vs. standard hydrogen electrode (SHE) per gram of Pt was taken as a gauge for electroactivity and some results are presented in Table 2. The ternary catalysts produced between 340-4300% more current per gram of Pt than Pt100/C, and the quaternary catalysts produced between 336-432% more current per gram of Pt than Pt70Ir15Ni15/C.
Pt70Ir10Sn15Ni5/C
|
Pt70Ir15Sn10Ni5/C
|
|
|
|
|
Pt70Ir15Ni15/C
|
Pt70Ir15Sn15/C
|
|
|
|
Figure 1: TEM Images of Selected Ternary and Quaternary catalysts.
Figure 2A: Cyclic voltammograms of anode electrocatalysts from ethanol oxidation at 25oC and scan rate of 100mV/s in 0.5M H2SO4 with 1M C2H5OH.
Figure 2B: Cyclic voltammograms of anode electrocatalysts from ethanol oxidation at 25oC and scan rate of 100mV/s in 0.5M H2SO4 with 1M C2H5OH.
Table 2: Summary of electroactivity of some electrocatalysts produced.
|
Electrocatalyst
|
Current @ 0.7 V vs. SHE (A/g Pt)
|
|
Pt100/C |
0.05 |
|
Pt70Ir15Ni15/C |
2.20 |
|
Pt70Ir15Cr15/C |
0.32 |
|
Pt70Sn15Ni15/C |
0.22 |
|
Pt70Ir15Sn10Ni15/C |
11.7 |
|
Pt65Ir15Sn10Cr10/C |
10.5 |
|
Pt65Ir15Sn10Ni10/C |
9.6 |
|
Pt70Ir15Sn10Cr5/C |
9.6 |
The activities of several quaternary catalysts in ethanol were evaluated using chronoamperometry at 0.6V vs SHE. Chronoamperogram of glassy carbon taken at the same voltage versus SHE was then digitally subtracted from the results obtained for the quaternary catalysts to correct for background currents. The results are presented in Figure 3. It is evident from Figure 3 that all PtIrSnNi quaternary catalysts combinations were initially effective in oxidizing ethanol based on the normalized current generated per gram of Pt. Pt70Ir10Sn10Ni10/C had the highest initial activity but was poisoned quickly. All of the catalysts examined eventually lost activity within a two hour period. Pt70Ir10Sn15Ni5 exhibited the longest lifetime.
|
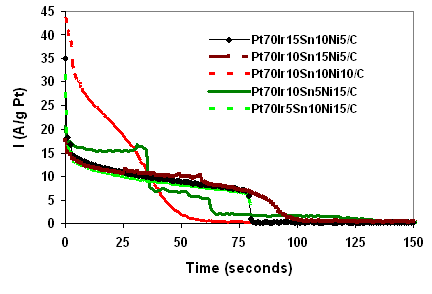
Figure 3: Chronoamperograms of different Wt% of quaternary catalyst PtIrSnNi/C subtracted from glassy carbon chronoamperogram in 0.5M H2SO4 and 1M C2H5OH at 25oC and scan rate of 20mV/s for 2 hours at 0.6V vs SHE.
Summary
Tertiary and quaternary catalysts were produced and evaluated for ethanol oxidation. Initial activity of the catalysts were significantly better than platinum alone. All catalysts examined exhibited a loss of activity (a.k.a. poisoning) with time. While these catalysts show promise, overcoming the poisoning effect of ethanol should be researched further.