Reports: AC3
47729-AC3 Abnormal Pincer-Type Carbenes for Functionalization of Hydrocarbons
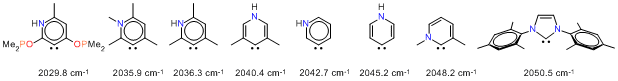
During the reporting period, much progress has been made involving computational and experimental work in our group. We are pursuing the synthesis of pincer ligands possessing a central pyridylidene fragment and their complexes with metals from Groups 8 and 9. In this project, we first evaluated the electron donor properties of several model carbenes (pyridylidenes) derived from pyridine. A comparison of DFT-calculated TEP values for 76 Ni(CO)3(NHC) complexes have established that pyridylidenes have electron donor properties significantly exceeding those of conventional' N-heterocyclic carbenes, e.g., such as IMes (Scheme 1). This study also demonstrated that C3-pyridylidenes (PyC3) are better donors than their C-2 and C-4 analogues, particularly when equipped with two phosphinite (-OPR2) groups at C-2 and C-4.
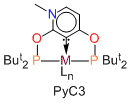
The exceptionally strong carbene donor properties of the PyC3 phosphinite in Scheme 2 attracted our attention and we decided to develop its synthesis and study the structure and reactivity of the corresponding PyC3 metal complexes (Scheme 2).
Scheme 2 Scheme 3
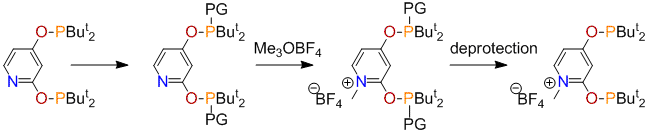
The phosphinite, 2,4-(OPBut2)2C5H3N, could be easily assembled from pyridine-2,4-diol and ClPBut2. Unfortunately, the resulting ligand coordinated with transition metals in a P,N-bidentate fashion and did not undergo metalation even after prolonged heating. To prevent formation of the N→M bond, two strategies were further explored, one (A) involving N-alkylation, and the other (B) employing steric hindrance by introducing a substituent at C-6. In A (Scheme 3), several methods of phosphorus protection were explored (PG = BH3, =O, =S, =Se). In all cases, except the selenide, the N-alkylated products, [2,4-(OP(PG)But2)2C5H3NMe]BF4, were obtained in excellent yields. Deprotection of these compounds, however, presented a challenge. With PG = BH3, the use of amines (NEt3, morpholine, N-methylmorpholine) unexpectedly resulted in decomposition, based on the observation of multiple peaks in the 31P NMR spectra of the reaction mixtures. Reductions of the oxides and sulphides (PG = =S, =O) with the help of Ranay Ni or silanes under different conditions were either inefficient or resulted in decomposition.
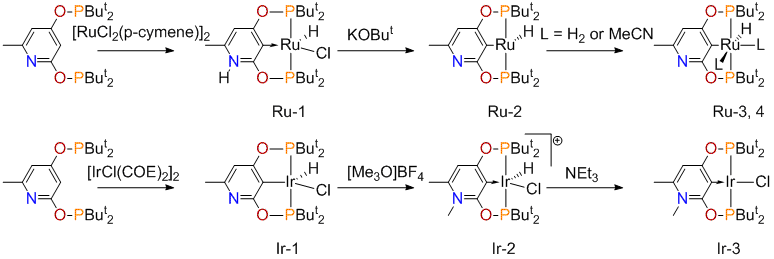
The alternative approach B turned out to be successful. The phosphinite, 2,4-(OPBut2)2-6-Me-C5H2N was obtained almost quantitatively from 6-methyl-pyridine-2,4-diol and ClPBut2. Reacting this ligand with Ru(II) and Ir(I) halides afforded the pincer complexes of Scheme 4, most isolated in good yields. These products have been structurally and spectroscopically characterized. We have evidence (NMR tube reactions) that rhodium complexes analogous to iridium compounds of Scheme 4 can be prepared as well. DFT calculations on complex Ru-1 provided a detailed electronic structure of this complex where the nitrogen and oxygen atoms donate π-electron density to stabilize the carbene carbon in a fashion similar to that found in conventional N-heterocyclic carbenes.
Scheme 4
Complexes of Scheme 1 have been tested in catalysis. Complex Ru-2 is active for ketone hydrogenation under H2 in the presence of KOBut (TON = 130, acetophenone). The active catalyst in this system is presumably the 14-electron hydride Ru-2. We expected that Ir-3 could be active for the Heck reaction, by analogy with the known palladium catalysts, PdCl[2,6-(OPR2)2C6H3]. However, in reaction of methylmethacrylate with bromobenzene only oligomerization of the olefin was observed. The 14-electron complex Ru-2 was tested in another important reaction: catalytic dehydrogenation of alkanes. Using the standard substrate, cyclooctane, we have observed formation of cyclooctene upon heating the catalytic solution in the presence of t-butylethylene.
Currently, we are developing a multi-gram scale synthesis of 2,4-(OPPri2)2-6-Me-C5H2N, a phosphinite possessing medium-size iso-propyl groups on phosphorus. Pincer complexes of this modified ligand are expected to show significantly enhanced reactivity and catalytic activity due to the reduced sterics around the central metal.
In summary, complexes Ru-1, Ir-2, and Ir-3 constitute first examples of pyridylidene pincer complexes possessing electron-rich metal centers and promising catalytic properties. Continuing experimental and computational studies and catalyst optimization are currently underway.