Reports: G10
48182-G10 Effects of Hydrogen-Surface Interactions on Photogenerated Electron Diffusion in Zinc Oxide Nanowires
The objective of our research is to study the interrelationship between impurity formation (including those on nanowires surfaces due to hydrogen-surface interactions), surface state energy levels, and photogenerated charge carrier diffusion in ZnO nanowires. The experimental approach is to correlate Raman scattering spectroscopy, scanning Kelvin probe microscopy, and scanning photocurrent microscopy techniques.
During this first year of the grant period (September 1, 2008 to August 31, 2009), we have made important progress on (1) using Raman scattering spectroscopy to identify impurity complexes and (2) implementing near-field scanning photocurrent microscopy techniques to directly measure charge carrier diffusion lengths in ZnO nanowires.
Identification of interstitial nitrogen molecules in ZnO nanowires
Raman scattering spectroscopy provides an effective approach to probing impurity incorporation in various materials. In particular, identifications of impurity species can be made via their vibrational frequencies. As impurity vibrational characteristics are sensitive to the surrounding environment, the lattice locations (e.g. interstitial vs. substitutional) of these impurity atoms can also be determined; such information is critical for evaluating the effects of impurities on material properties.
 Using
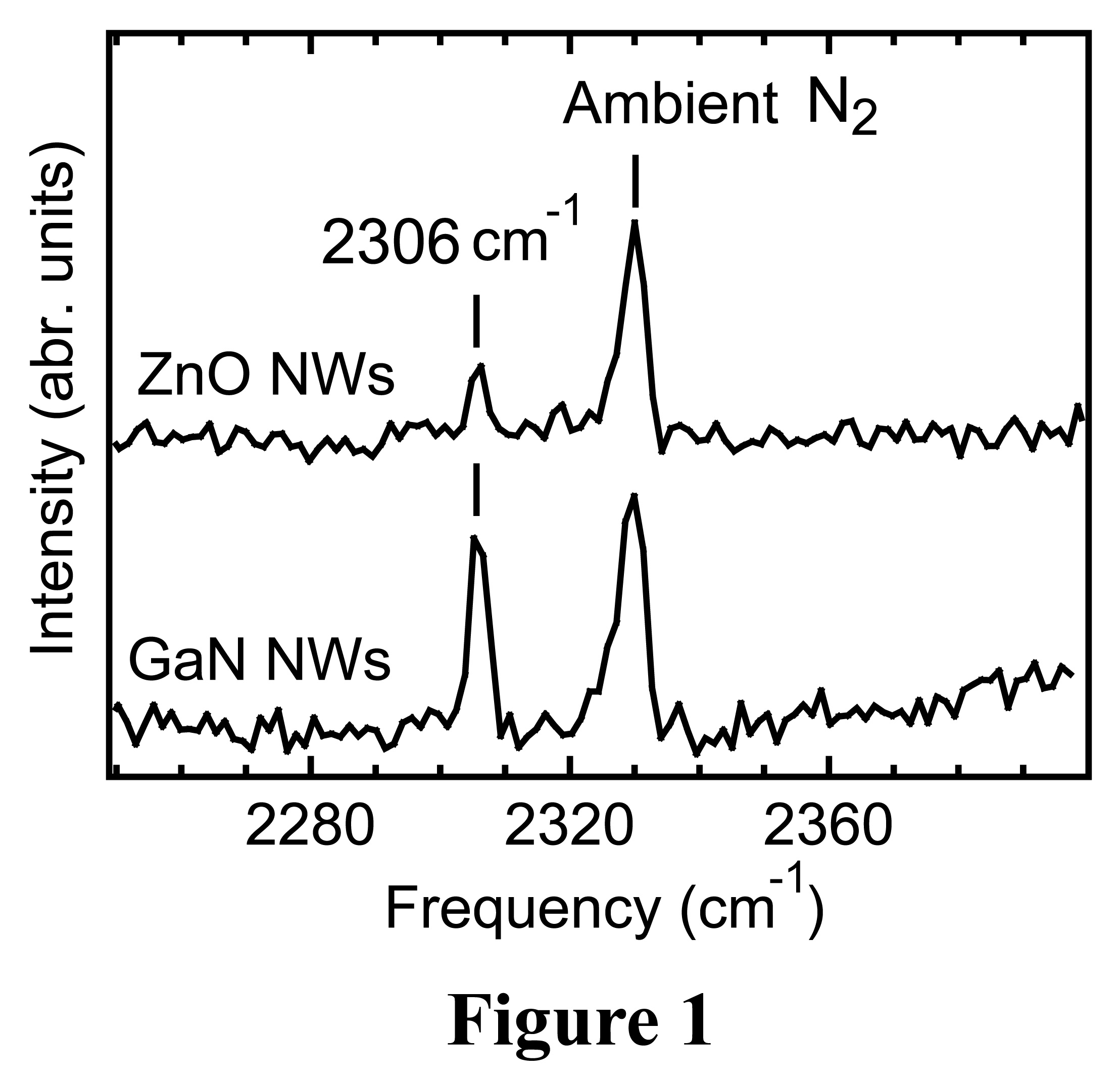
the Raman scattering spectroscopy technique, we observed an impurity
vibrational mode at ~ 2306 cm-1 (Figure 1). This vibrational mode
has been observed in intentionally nitrogen-doped bulk ZnO and attributed to
nitrogen-related complexes. As the vibrational frequency of this mode is very
close to that of nitrogen molecules (~2330 cm-1), we assign this
peak to the vibrational mode of interstitial nitrogen molecules in ZnO
nanowires. This is consistent with the absence of this mode in infra-red
absorption spectra (not shown here), as nitrogen molecules are Raman active but
infra-red inactive. The presence of interstitial nitrogen molecules inside ZnO
nanowires is further confirmed by laser-ablation mass spectroscopy measurements
(not shown here).
Using
the Raman scattering spectroscopy technique, we observed an impurity
vibrational mode at ~ 2306 cm-1 (Figure 1). This vibrational mode
has been observed in intentionally nitrogen-doped bulk ZnO and attributed to
nitrogen-related complexes. As the vibrational frequency of this mode is very
close to that of nitrogen molecules (~2330 cm-1), we assign this
peak to the vibrational mode of interstitial nitrogen molecules in ZnO
nanowires. This is consistent with the absence of this mode in infra-red
absorption spectra (not shown here), as nitrogen molecules are Raman active but
infra-red inactive. The presence of interstitial nitrogen molecules inside ZnO
nanowires is further confirmed by laser-ablation mass spectroscopy measurements
(not shown here).
The source of this unintentional nitrogen
incorporation is most likely the residual background nitrogen gas in growth
reactors. The catalytic vapor-phase transport method, one of the most common
techniques for nanowire synthesis, is usually implemented in low-pressure (~
several mTorr) or atmospheric chemical vapor deposition (CVD) systems. In these
CVD chambers, residual background gases (e.g. N2, O2, and
H2) are often present. We note that nitrogen molecules dissolve
easily in molten metals and become atomic nitrogen. This process is expected to
become more efficient with the large surface area of metal nanocatalysts. These
dissolved nitrogen atoms then diffuse into nanowires during the growth and form
interstitial molecules. This incorporation route should lead to an uniform
distribution of interstitial nitrogen molecules along nanowires. While the
Raman intensity of this vibrational mode is rather weak in ZnO nanowires, GaN
nanowires grown under similar conditions exhibit much stronger signals (Figure
1),
this allows us to verify this proposed nitrogen incorporation route via
spatially resolved Raman studies.
 and
In particular, ammonia (NH3), a
precursor for GaN nanowire growth, also easily dissolves in metals and
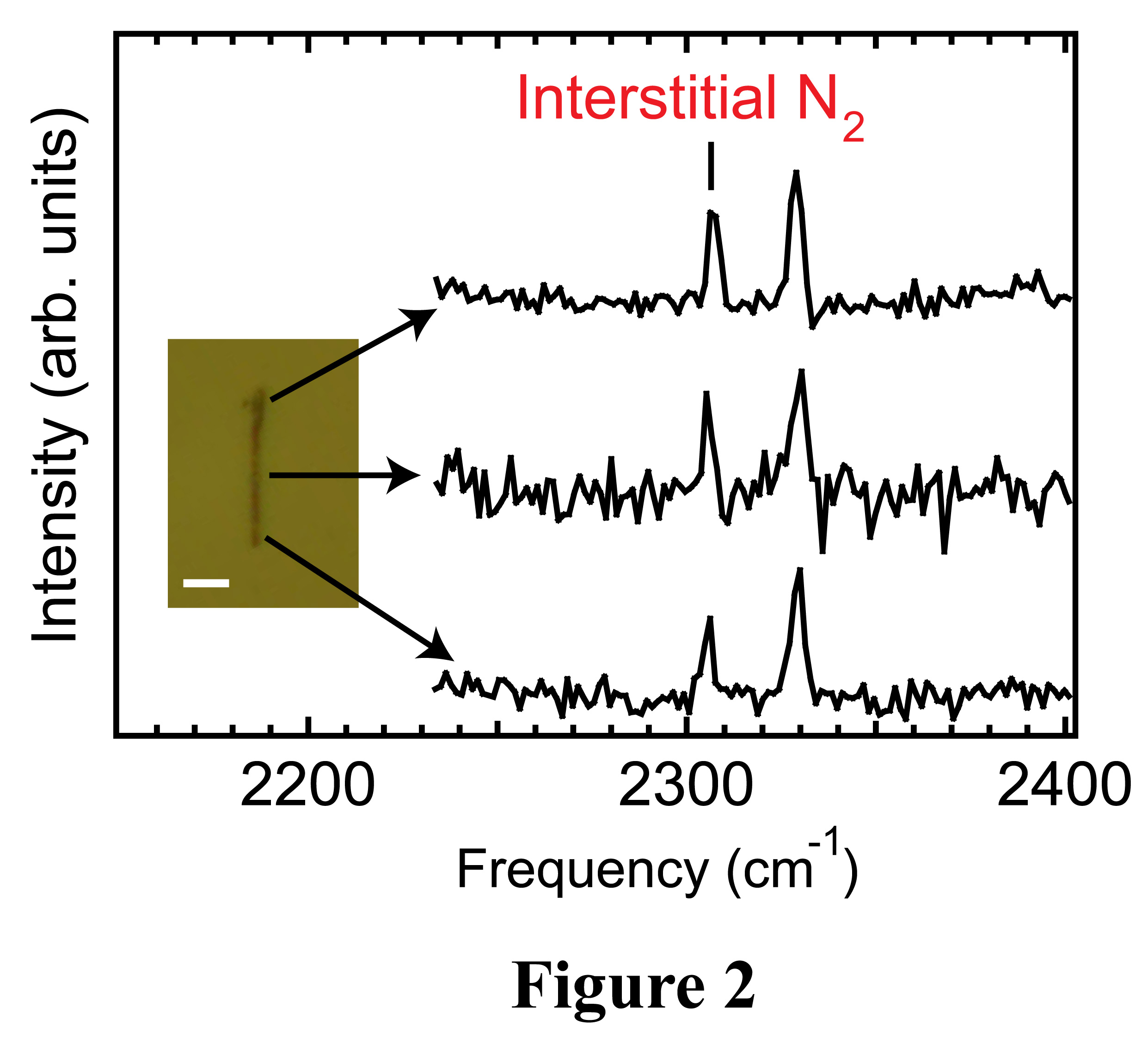
decomposes into nitrogen atoms. As can be seen from spatially-resolved Raman spectra
of a single GaN nanowire [Figure 2, with an optical image of this nanowire
shown in the inset (scale bar: 5µm)], the interstitial molecular nitrogen
vibrational mode does not show significant intensity variations along the
nanowire, indicating that nitrogen incorporation via the catalysts is the
dominant route.
and
In particular, ammonia (NH3), a
precursor for GaN nanowire growth, also easily dissolves in metals and
decomposes into nitrogen atoms. As can be seen from spatially-resolved Raman spectra
of a single GaN nanowire [Figure 2, with an optical image of this nanowire
shown in the inset (scale bar: 5µm)], the interstitial molecular nitrogen
vibrational mode does not show significant intensity variations along the
nanowire, indicating that nitrogen incorporation via the catalysts is the
dominant route.
Interstitial nitrogen molecules can form mid-gap energy states in ZnO and thus can significantly modify material properties, including charge carrier transport characteristics. It is therefore critical to investigate the effects of interstitial nitrogen molecules on carrier diffusion in ZnO nanowires. In addition, as hydrogen can passivate various impurities/defects in semiconductors, it would be also interesting to explore the effects of hydrogen incorporation. Furthermore, we believe that our findings of unintentional nitrogen incorporation are broadly relevant. In particular, residual nitrogen molecules may be present in most low-pressure and atmospheric nanowire growth reactors. Additionally, the incorporation of nitrogen atoms via metal nanocatalysts might be a universal phenomenon during the metal-catalyzed nanowire growth process. These results have been published in Nano Letters.
Direct measurement of charge carrier diffusion length in ZnO nanowires
We have implemented the near-field scanning
photocurrent microscopy (SPCM) technique for the direct measurement of carrier
diffusion length in single ZnO nanowires. In particular, we have fabricated
Schottky diodes based on single ZnO nanowires, using the Schottky contact as
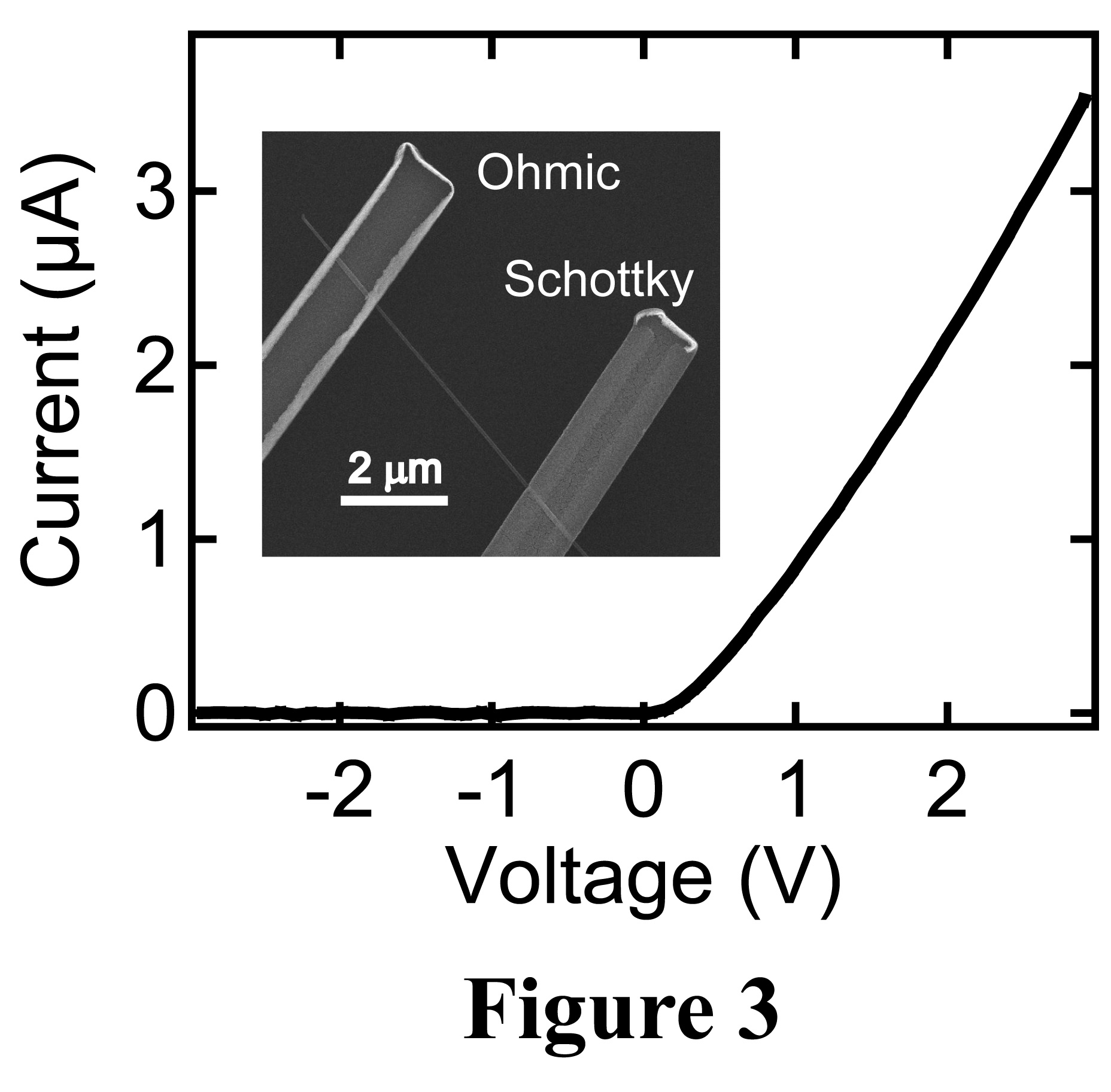
the efficient photogenerated carrier collector. The inset to Figure 3 shows a
Schottky diode with Pt (Ti/Au) as the Schottky (Ohmic) contact. The nature of
these contacts is confirmed by the rectifying current-voltage
characteristics
(Figure 3) with the Pt as the biasing contact.
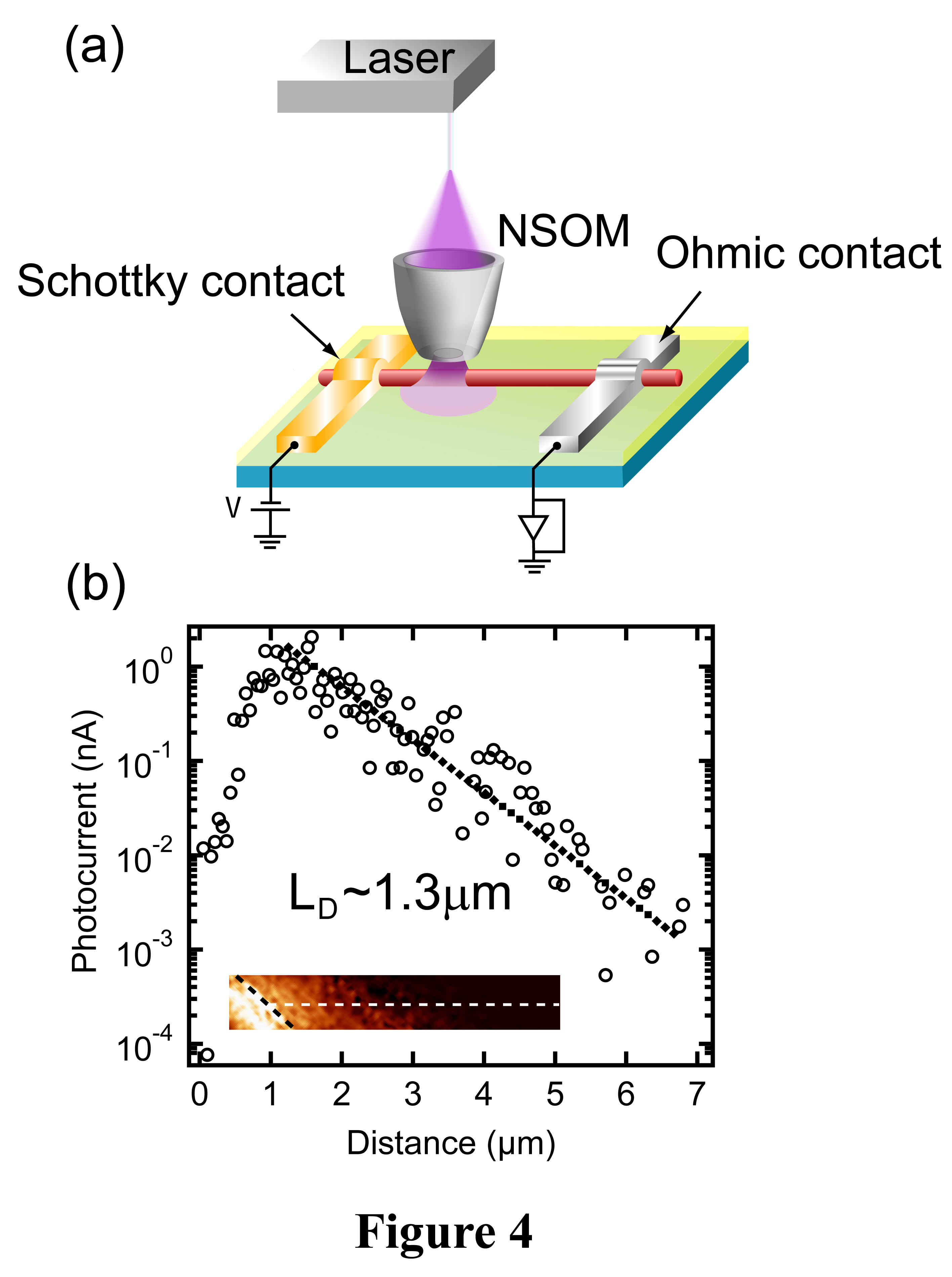
The schematic SPCM setup is shown in Figure 4 (a). As a near-field scanning optical probe moves closer to the Schottky junction, the photogenerated carriers (holes in this case) begin to diffuse into the space charge region and are collected by the metal electrode. This induces a photocurrent, the magnitude of which is directly proportional to the number of carriers reaching the space charge region. Therefore, the photocurrent spatial profile represents the carrier spatial distribution (a single exponential), from which the carrier diffusion length (LD) can be extracted. A typical photocurrent image is shown in the inset to Figure 4 (b), with the black (white) dashed line representing the Schottky electrode and the nanowire. The spatial profile of the photocurrent (open circles) close to a Schottky contact is plotted in Figure 4(b), and the fitting using a single exponential (the dashed line) yields LD of ~ 1.3µm.
Plans for the second year of the grant period (September 1, 2009 to August 31, 2010)
In the second year of the grant period, we plan (1) to use Kelvin probe microscopy techniques to investigate the nanowire surface states; (2) to study the effects of hydrogen on the surface state formation as well as the interstitial nitrogen molecule incorporations; and (3) to examine how the carrier diffusion length changes with the presence of hydrogen on the surface and inside the crystal lattice.