Reports: G1
47843-G1 Development of Chiral Lewis Base as Novel Nucleophilic Catalysts and Their Application in Asymmetric Synthesis
Narrative report:
Our goal in this proposed research is to develop new Lewis base (nucleophilic base) and novel chemical transformations that convert petroline products into other higher value chemicals. During the last year, we have been focused on the Lewis based catalyzed cross conjugated addition. To our great please, simple proline was identified as a novel Lewis base in promoting nitroalkene activation. This discovery revealed an novel reaction path way, where conventional Lewis base catalyst, such as DMAP, DABCO, can not promote the transformations due to the fast polymerization of activated intermediate (Scheme 1).
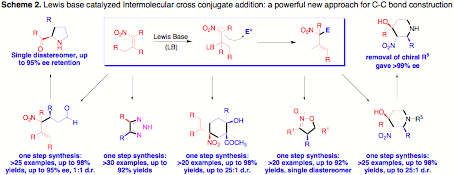
Application of this new strategy, several different electrophiles were applied to reactive the Lewis based activated nitroalkene and a series new reactions we discovered, resulting in 9 publication so far. Several complex organic molecules were prepared through very simple starting materials in one pot with good to excellent yield and good stereoselectivity (Scheme 2).
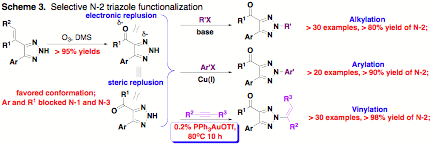
Moreover, the discovery of one-step synthesis of 4,5-disubstituted-1,2,3-NH-triazoles provided an alternative for the synthesis of these very attractive heterocycles which were difficult to be prepared using other approaches, such as "click-chemistry". With effective conformational control, the regio selective N-2 functional triazoles were successfully prepared through post triazole derivatization (Scheme 3).
With these diverse functional triazoles in hand, we then investigated their coordination ability to different transition metals. Various triazole bound complexes were successfully prepared, including Rh, Au, Ir and BH3 complexes. This new class of metal complexes has revealed unique reactivity and further investigation of their catalytic reactivity are currently under investigation.