Reports: GB3
47823-GB3 Disruption of Metal-Metal Interactions and Extended Linear Chains by Organic Solvent Molecules
1. Introduction
A unique feature of some of the group 10 and 11 metal ions is their fascinating ability to assemble in an extended low dimensional networks. The extended metal-metal interactions, as reported in numerous examples, proceeds in ligand unsupported fashion. Pt(II), Au(I), and Ag(I) in particular are known to exhibit this behavior, where the weak metal-metal interaction leads to aggregation and formation of dimeric and extended chain systems [4-9]. Earlier studies on Pt(II) tetra cyano complexes demonstrated the presence of pseudo-1-D columnar chains4a,6 of planar anions stacked parallel to one another. The significant Pt-Pt interactions that occur between adjacent Pt tertracyano anions in these chains exhibit highly tunable spectral properties, where the Pt-Pt distances, and hence the associated spectroscopic properties, can be tuned by chemical and physical variations such as choice of counter cation, applied pressure, and solvent variations. The Au(I) and Ag(I) complexes also exhibit similar dependency, although they involve closed-shell, diamagnetic d10 metal centers, and consequently no apparent valence electrons available to connect them
The fund from the PRF program has allowed us to continue investigating factors that influence the extent of metal-metal interactions in these systems. In addition, the PRF fund has also been invaluable to our research group with respect to establishing a new area of work especially in directions we had not initially anticipated. We have been able to use this support to begin collaborative work involving dual-donor sensitization with the Sykora's group at the University of South Alabama.
2. Results
a) We
are actively pursuing synthetic procedure that will help us establish
model compounds containing only two metal atoms. As
a starting point, we are actively developing bidentate bridging ligands that
will restrict and/or limit polymerization and extended chain formation. The
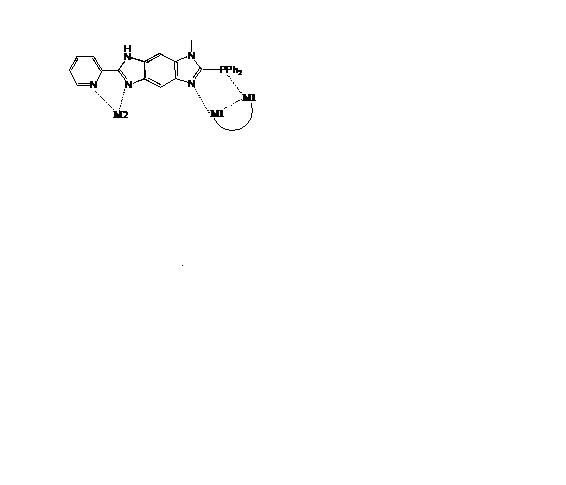
targeted ligands are based on benziimidazole, where the diphenyl phosphino
side, with a bite distance and angle of about 2.85 Å and ~80.5°, respectively,
is an ideal site to bring two metal centers in close proximity.
Metal complexes of the dppbim
ligand are expected to show a broad range of properties, including emission
behavior that can be influenced by the extent of M-M interaction [16, 17]. This
research is expected to increase the detailed, fundamental understanding of the
interaction between organic solvent molecules and the gold-phosphine complexes.
We have successfully completed the phosphorylation, and purification steps and
have started coordinating the ligand with metal ions. The main goal is to
establish correlation between the metal-metal interaction and the
photoluminescence properties of the resulting complexes.

b) The second
part of our project involves fundamental understanding of emission enhancement
through dual donor sensitization. This is a new area that our group initiated
in collaboration with Sykora's group at the University of South Alabama. We
believe the use of multiple donors for systematic design of luminescent
lanthanide probes is an area that needs to be developed since an
understanding of the basic mechanisms
of emission enhancement through a cooperative effect may lead to a rational
design of materials useful for various applications.
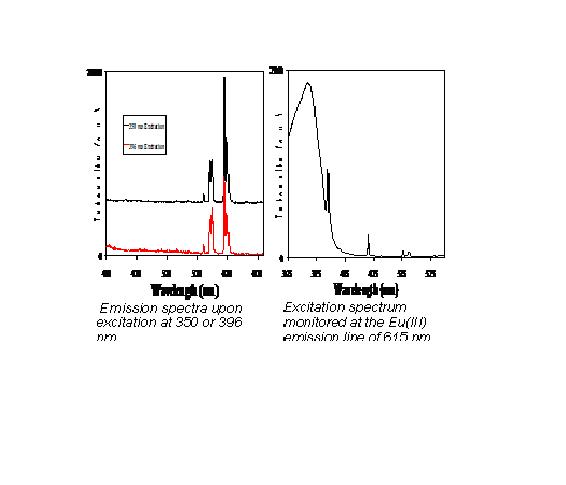
In our preliminary work in this area we have chosen two donor systems that both absorb in the UV region and undergo energy transfer processes to selected lanthanide ions. The chosen systems involve the tetracyanoplatinate (TCP) and the terpyridine ligand directly coordinated to the lanthanide ion as donors. Three different europium tetracyanoplatinates all incorporating the terpyridine ligand have been synthesized. Variation of the counter anion in the Eu(III) starting materials provided isolation of three distinct structure types. One-dimensional polymeric structure types were exhibited in two of the three compounds, which were prepared in reactions utilizing Eu(III) nitrate or Eu(III) triflate. The 3rd compound revealed a zero-dimensional ionic compound containing two [Eu(C15H11N3)(H2O)2(CH3COO)2]+ cations and one Pt(CN)42- anion. The structural differences provided the opportunity to investigate the photoluminescence properties of several related systems with differing coordination environments of the Eu(III) ion. It was revealed that the sensitization phenomenon varies drastically due to the change in the structural morphology. This work resulted in two publications so far and several others to follow.