Reports: AC6
46311-AC6 Kinetics, Reaction Mechanisms, and Relative Stabilities of Heterocyclic Ring Molecules in Ionized Environments
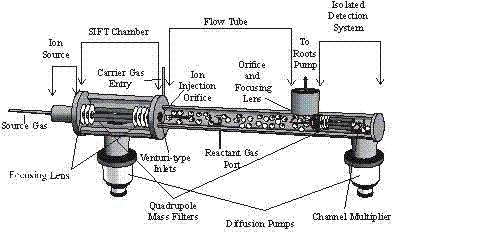
Hydrocarbon ions and neutrals are known to be important in combustion, supersonic ram jets, in molecular synthesis in interstellar gas clouds, and in the chemistry of planetary atmospheres (such as that of Titan, a planetary satellite of Saturn). Single ring hydrocarbons have been detected in some of these media and they are particularly interesting chemically because of the possibility of aromaticity and anti-aromaticity with and without heteroatoms in the ring (pyridine, C5H5N; pyrimidine, C4H4N2; piperidine, C5H11N; 1-4 dioxane, C4H8O2; benzene, C6H6; cyclohexane, C6H12 ; toluene, C7H8, all six-membered rings have been studied), and the five-membered rings, pyrrole, C4H5N; pyrrolidine, C4H9N; furan, C4H4O and tetrahydrafuran, C4H8O. Important in hydrocarbon plasmas are the stable ion species CH3+ and C3H3+ and the reactivity of these with the rings listed above have been investigated in our Selected Ion Flow Tube.
Figure 1. Almost none of these reactions had been studied previously. The reactions with CH3+
have shown reactivity by charge transfer (dissociative and non-dissociative),
proton transfer, H-atom abstraction and surprisingly association. Association was not expected to be
competitive with the other processes and indeed does not compete when the ring
is anti-aromatic with no p electrons in the ring.
However, with p electrons in the ring, association is a very
significant channel. From theoretical
studies, the mechanism is believed to proceed rapidly initially by attraction
of CH3+ to the p electrons and then with
an isomerization in which the CH3+
attaches to a carbon in the ring, displacing the H-atom to the para position.
Following this, it was expected that C3H3+
might behave similarly, however this is not the case. C3H3+ also
has an additional complication that two isomers were detected, the cyclic cyclopropenyl and the acyclic propargyl
cations. These ions were produced in a ~75% to ~25% cyclic to acyclic ratio by
electron impact on propyne, C3H4, in a low pressure ion
source. Examples were seen where the
two isomers reacted at the same rate and where the cyclic form reacts much more
slowly.
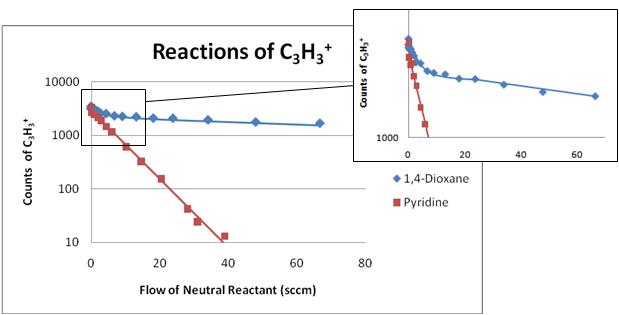
Figure 2. The figure above shows the reaction of C3H3+
with two different neutrals, 1,4-dioxane and
pyridine. In the reaction with 1,4-dioxane the acyclic isomer reacts at a much faster rate
than the cyclic isomer whereas with pyridine both isomers react at the same
rate.
Association is still an important product channel but was
seen both with aromatic and anti-aromatic rings. For the hydrocarbon six membered rings, (C6H6,
C6H12, C7H8), the cyclic form of
the ion reacts slowly. For the acyclic
form of the ion, the reactions are gas kinetic and association is an important
channel for the aromatic rings. No
association was observed for the anti-aromatic C6H12. For reactions of both cyclic and acyclic ions
with nitrogen containing heterocycles, both the
aromatic and anti-aromatics (C5H5N, C4H4N2,
C5H11N) react rapidly and in all
cases association is a very important channel (76 to 89%) even for the
anti-aromatic C5H11N.
For the anti-aromatic with oxygen in the ring (C4H4O2),
the acyclic C3H3+ reacts rapidly, and the
cyclic slowly, but association is again seen.
For the five membered rings (C4H5N, C4H9N,
C4H4O, C4H4O) the rates of the
reactions are variable as are the amounts of association with no particular
trends through the data. Reactions of other hydrocarbon ions, C3H+,
C3H2+ and C3H4+,
were also studied. For these ions, the
anti-aromatic reactions are generally slow as is that of toluene in the
aromatics. Reactions with the five
membered rings show no particular trends except that C3H4+
reactions seem to react somewhat slower than the other ions.
This award has enabled us to study the mechanisms of
ion-molecules reactions of compounds, which are important in hydrocarbon
plasmas of petroleum species. The data
obtained have permitted us to successfully write proposals relevant to
applications in the interstellar medium and the Titan planetary satellite
atmosphere.
The students funded from this award have learned a great
deal about the kinetics of ionic reactions important in a variety of plasma
environments. They have learned how to
operate complex apparatuses (Selected Ion Flow Tubes and Flowing Afterglows)
for obtaining these kinetics and for understand
reaction mechanisms. One student has
graduated with a Ph.D and one other has almost
completed her PhD. More than 57
reactions have been studied. The
students have written two papers and three more are in preparation.