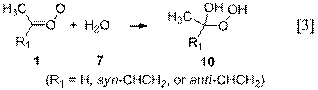Reports: B6
44764-B6 Computational Studies of the Atmospheric Chemistry of Alkene Ozonolysis Intermediates
My laboratory has focused on two projects with PRF support. Both have involved the use of quantum chemistry and statistical rate theory to predict quantities that can be tested against experiment.
(1) The Ozonolysis of Trans-2-Butene. My students and I used highly accurate electronic structure calculations, combined with master equation simulations, to predict the outcome of trans-2-butene ozonolysis in the atmosphere. Figure 1 summarizes our results.
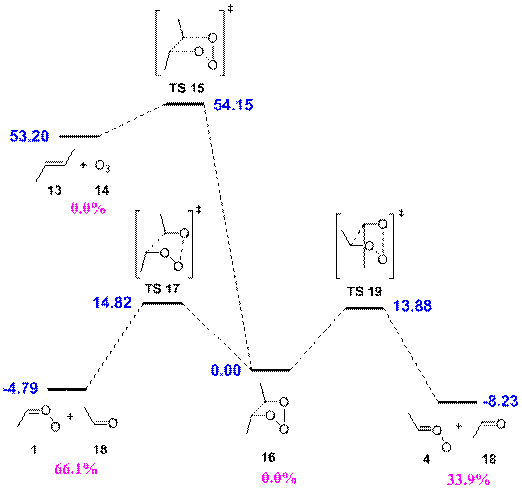
Figure 1. Trans-2-butene ozonolysis mechanism treated by RRKM/master equation simulations. The MCG3//QCISD/6-31G(d) relative energies (at 0 K, in kcal mol-1) are given in blue, and the 1-atm pseudo steady-state yields for the well and exit channels are given in purple.
The formation of the primary ozonide (16) was the entrance channel, and the initial energy of 16 was represented by a shifted thermal (298 K) distribution truncated at the zero-point corrected energy of transition structure TS 15. The exit channels were the formation of syn acetaldehyde oxide 1 (and acetaldehyde, 18), formation of anti acetaldehyde oxide 4 (and acetaldehyde, 18), and reversion to trans-2-butene (13) and ozone (14). There was no variation in pseudo steady-state yields from 1 Torr up to 1 atm; the 1-atm yields are shown in Figure 1. None of the primary ozonide is predicted to revert to reactants or be collisionally stabilized. A large majority (66%) of 16 decomposes to the syn conformer of the Criegee intermediate. This is in spite of the fact that the MCG3//QCISD/6-31G(d) barrier to forming the syn conformer is ~0.9 kcal mol-1 than the barrier to forming the anti conformer. The preference for the syn conformer arises from the large number of states available to the chemically activated 16 at the syn transition state (TS 17), particularly from the active K-rotor of TS 17.
We then simulated possible reactions of the Criegee intermediates formed upon primary ozonide cycloreversion. The parts of the mechanism that we considered, and the relative energetics of all participating species, are depicted in Figure 2, and master equation results are summarized in Figure 3.
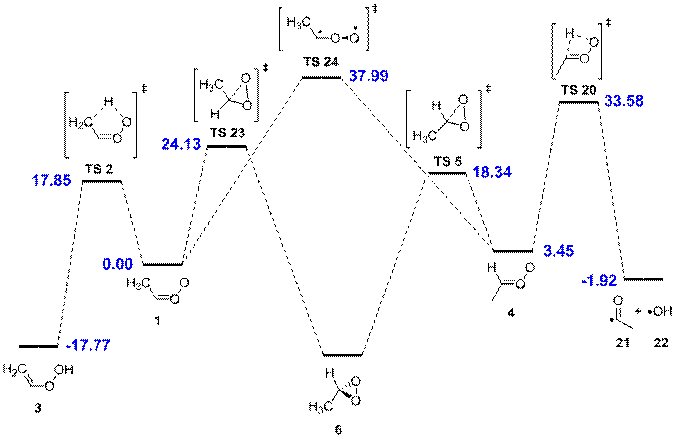
Figure 2. Acetaldehyde oxide mechanism treated by RRKM/master equation simulations. The MCG3//QCISD/MG3 relative energies (at 0 K, in kcal mol-1) are given in blue.
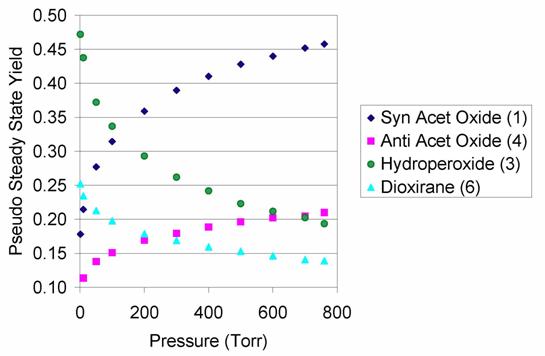
Figure 3. Product
yields of the trans-2-butene
ozonolysis reaction, as predicted by RRKM/master equation simulations. Each simulation was run for 103
collisions to ensure that the pseudo steady state was achieved. Trials were run at pressures of 1, 10,
50, 100, 200, 300, 400, 500, 600, 700, and 760 Torr. Each pseudo steady-state yield reported
is the average result of 105 We
predict that the branching between prompt isomerization (leading to 3 and 6) and thermalization (leading to 1 and 4) depends
significantly on pressure. At 1
atm, almost 70% of the Criegee intermediate formed in trans-2-butene ozonolysis is thermalized. The subsequent reactivity of 1 and 4 was the subject of our other major project.
Table 1 summarizes our
predictions at the range of temperatures found in the troposphere. All reported rate constants include the
effect of tunneling. We determined
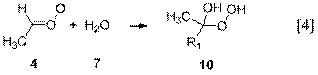
pseudo-first-order rate constants for hydration reactions 3 and 4 by assuming
that the equilibrium vapor pressure of water was present at a given temperature.
TABLE 1. First-Order
and Pseudo-First-Order Rate Constants for Criegee Intermediate Reactions
CVT rate constant (s-1)
200 K
250 K
298 K
species 1 (R1 = H)
reaction 1
9.70 x 10-2
1.48 x 100
2.42 x 101
reaction 3
6.22 x 10-10
1.19 x 10-5
5.57 x 10-3
species 1 (R1 = syn-CHCH2)
reaction 1
9.17 x 10-1
1.12 x 101
1.46 x 102
reaction 3
1.41 x 10-8
9.56 x 10-5
2.64 x 10-2
species 1 (R1 = anti-CHCH2)
reaction 1
4.97 x 10-2
8.27 x 10-1
1.47 x 101
reaction 3
2.47 x 10-10
2.73 x 10-6
1.25 x 10-3
species 4
reaction 2
2.03 x 10-4
4.51 x 10-1
6.72 x 101
reaction 4
2.92 x 10-3
3.03 x 100
2.21 x 102
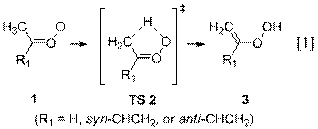
Syn-alkyl Criegee intermediates
isomerize via a 1,4-hydrogen shift (Reaction 1) between 3 and 8 orders of
magnitude faster than they react with water (Reaction 3). The product of the isomerization, a
vinyl hydroperoxide (3), falls apart
promptly under atmospheric conditions to afford hydroxyl radical (•OH). The significance of our finding is that
the •OH yield from the ozonolysis of a given alkene should be determined
only by the yield of syn-alkyl
Criegee intermediate (such as what is shown in Figure 1 above). For the case of trans-2-butene, we predict an •OH yield of 0.651, which
agrees with the experimental consensus that the •OH yield lies between 0.52 and 0.67.
Anti-alkyl Criegee intermediates react
far more rapidly with water (Reaction 4) than do the syn conformers, and the hydration reaction can be comparable or
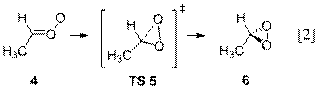
faster in rate than the isomerization (Reaction 2) to form dioxirane (6). The product of hydration is a saturated
hydroperoxide (10), which will not
decompose under atmospheric conditions to form •OH. Our results also
suggest that other common Criegee intermediate scavengers, such as aldehydes
and SO2, react almost exclusively with the anti conformer. The
yield of scavenger products (secondary ozonides for the aldehyde reaction, and
H2SO4 for the SO2 reaction) is therefore an
underestimate of the total yield of thermalized Criegee intermediate formed in
an ozonolysis reaction.