Reports: GB1
48256-GB1 Enantioselective Nucleophilic Catalysis for the Synthesis of beta-Lactams and other Nitrogen Containing Heterocycles from Isocyanates
The goals of this project were originally envisioned as three-fold: (1) the development of a nucleophile catalyzed isocyanate-olefin cyclization to make β-lactams (2) an asymmetric version of the reaction and (3) the catalytic asymmetric synthesis of other heterocycles and extension toward a broad research program rooted in nucleophilic catalysis. Although we have made progress toward the first goal, much of the significant progress over the past year has been toward goal 3 and we have initiated a broadly defined program centered on nucleophilic catalysis and the synthesis of heterocycles and carbocycles.
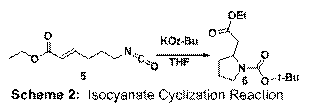
Our work began with the synthesis of substrates that consist of both an isocyanate and an electron poor alkene to test the intramolecular cyclization reaction on route to synthetically and medicinally valuable β-lactams. Cyclization substrate 5 was successfully prepared in four steps (Scheme 1).
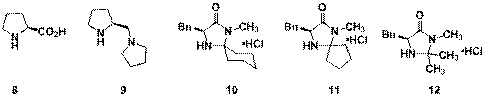
The key cyclization was attempted on substrate 5 using a variety of well known nucleophilic catalysts. Unfortunately, attempts to promote the desired cyclization via nucleophilic catalysis did not result in the formation of bicyclic β-lactams. Evidence exists (from NMR) for the initial attack of the nucleophile into the isocyanate, however the key conjugate addition step has not taken place. We have, on the other hand, used an anionic nucleophile to effect the key C-N bond forming event. Treatment of 5 with KOt-Bu led to the formation of known pyrrolidine 6 (Scheme 2). One current project is aimed at the cyclization of 5 using stoichiometric nucleophiles. We will use chiral anions to in efforts to achieve asymmetric syntheses of 2-substituted pyrrolidines which are found in innumerable natural products.
In order to make the conjugate addition of the zwitterion a more favorable process, a stronger electron withdrawing group than the ethyl ester group may be needed. We have begun efforts aimed at preparing isocyanates tethered to more electron poor alkenes including nitroalkenes, unsaturated nitriles, and ketones. Following our original plan, olefination of lactol 2 should provide the requisite alkene analogous to 3 and amenable toward further conversion to the isocyanate. However, in all the previously mentioned cases, premature cyclization of the tethered OH group led to the formation of pyrans such as 7 (Scheme 3).
Current efforts are aimed at taking advantage of this premature cyclization. We will explore the use of chiral amine catalysts in the synthesis of 2-substituted pyrans which are abundant in natural products and pharmaceuticals. A variety of known chiral amine catalysts have already been screened under several reaction conditions. In each case, there was a small but noticeable increase in rate compared to uncatalyzed reactions. Unfortunately, enantioselectivities were low in all examples.
|
solvent
|
temp
|
time
|
catalyst
|
% yield
|
%ee
|
|
benzene
|
70
|
5 d
|
None
|
73
|
<5
|
|
benzene
|
70
|
5 d
|
8
|
80
|
<5
|
|
benzene
|
70
|
47 h
|
9
|
53
|
<5
|
|
benzene
|
70
|
3 d
|
10
|
53
|
<5
|
|
benzene
|
70
|
2.5 d
|
10 (free base)
|
90
|
<5
|
|
THF/H2O
|
RT
|
5.5 d
|
11
|
30
|
<5
|
|
THF/H2O
|
RT
|
5.5 d
|
12
|
30
|
<5
|
|
THF/H2O
|
70
|
30 h
|
12
|
70
|
~7
|
The results above indicate that iminium catalysis may not be operating. This project is now focused on using aldehyde Wittig reagents. The aldehyde intermediate resulting from the Wittig reaction should be more reactive toward amine catalysts and therefore more likely to cyclize under catalytic conditions. We also expect a greater steric differentiation between the two groups on the carbonyl which may lead to increased enantioselectivites.
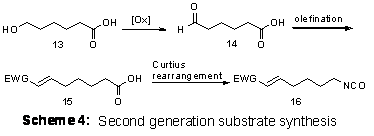
Future efforts continue toward the synthesis of β-lactams using nucleophilic catalysis. Plans include new routes toward cyclization precursors with stronger electron withdrawing groups following the general plan outlined in Scheme 4.
We have also undertaken studies on the intramolecular Morita-Baylis-Hillman reaction which was prompted by our interest in nucleophilic catalysis. The Morita-Baylis-Hillman reaction is often quite slow resulting in the need for highly reactive alkyl phosphines and high catalyst loadings. To address this, a variant of the transformation is being investigated where the initial electrophile is an alkyne (Scheme 5). In this process, an external pronucleophile is required to regenerate the phosphine catalyst.
In a preliminary experiment, 17 was treated with catalytic n-Bu3P and 1.5 equivalents of TMSCN resulting in a rapid reaction. Following treatment with aqueous CsF, the product 18 was isolated in 61% yield. This encouraging result has led to an effective method to construct a variety of highly functionalized carbocycles similar to 18.