Reports: AC10
47077-AC10 Bioinspired Hybrid Systems for the Photoelectrocatalytic Generation of Solar Hydrogen and Fuels
The photoelectrochemical generation of hydrogen or hydrocarbons and oxygen from water is a viable alternative to photocatalytic and solely electrochemical approaches. The key to this technology is finding ways to generate long-term stable and inexpensive materials for photoelectrochemical electrodes. We have focused our research on photocathodes for the reduction of carbon dioxide and water.
They consist of the following components:
1) A non-conductive glass plate, which is used as carrier
2) A layer of epoxy glue containing 1.2 percent (by weight) of silver microparticles (d=1.45±0.2 µm). This layer has two functions: a) firmly attaching the ruthenium-complex/titanium dioxide particles and b) providing an electrical connection.
3) Nanostructured ruthenium-complex/titanium dioxide particles, prepared via a sol/gel procedure from ruthenium(II)quaterpyridinium complexes and Ti(O-Bu)4.
4) Palladium-nanoparticles (d=54±18nm) have been deposited onto the composite electrodes by electrochemical reduction of PdCl2 from aqueous medium.
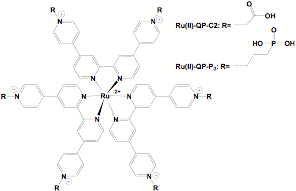
We have used two ruthenium-quaterpyridinium complexes, which were synthesized in our laboratory: Ru(II)-QP-C2 and Ru(II)-QP-P3.
Figure 1: Ruthenium-Quaterpyridinium complexes used for the synthesis of sensitizer-containing titanium dioxide gels. The carboxylic acid (Ru(II)-QP-C2) and phosphonate groups (Ru(II)-PQ-P3) bind to TiO2 and thus become incorporated into the inorganic nanogels.
Table 1 illustrates the redox potentials of CO2 to produce various hydrocarbons at pH 7. According to the reduction potential given in Table 1, it is almost impossible to do one electron reduction of CO2. According to the reduction potentials, multi-electron reduction reactions of CO2 look thermodynamically favorable. Reduction potential of CO2 to CH4 is even smaller than the reduction potential of H+ to hydrogen. Although CO2 to CH4 is more favorable than the reduction of H+ to hydrogen (thermodynamically), CO2 to CH4 reduction kinetically is difficult due to the requirement of multi-electrons for the reduction. However, to reduce CO2 successfully to CH4 and H2O, the catalyst should be able to undergo efficient multi-electron and multi-proton transfer reactions.
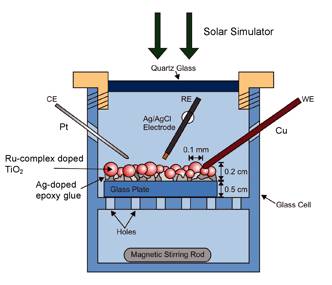
The new electrode materials have been tested in a photoelectrochemical cell, which is shown in Figure 2.
Figure 2: Photoelectrochemical cell: RE: reference electrode (Ag/AgCl 0.230±10 mV), WE: working electrode (Cu), CE: counter electrode (Pt). A mercury high-pressure lamp (150 W, Oriel 68806) was used to simulate sunlight. The electrodes were immersed in a 5.0 x 10-2 M NaHCO3 solution, pH=7.2.
The successfully prepared electrodes were investigated by using differential pulse voltammetry (DPV, see Figure 1 for the experimental setup). The electrode was placed on a perforated glass plate. The composite electrodes were contacted with a copper wire. A Pt wire was used as counter electrode. As reference electrode, an Ag/AgCl electrode was employed.
All manufactured electrodes showed principally the same peaks. They were identified as reduction peaks of various carbonate reduction processes and the hydrogen reduction reaction, as summarized in Table 1.
Table 1: Standard Redox-Potentials of various carbonate reduction processes and the hydrogen reduction reaction (vs. standard hydrogen electrode).
|
Reaction
|
Reference E0 / V (vs SHE)
|
|
CO2 + 1 e- → CO2∙-
|
approx. - 2
|
|
CO2 + 2 H+ + 2 e- → HCOOH
|
-0.61
|
|
CO2 + 2 H+ + 2 e- → CO + H2O
|
-0.52
|
|
CO2 + 4 H+ + 4 e- → HCHO + H2O
|
-0.48
|
|
2 H+ + 2 e- → H2
|
-0.41
|
|
CO2 + 6 H+ + 6 e- → CH3OH + H2O
|
-0.38
|
|
CO2 + 8 H+ + 8 e- → CH4 + 2 H2O
|
-0.24
|
|
CO2 + 4 H+ + 4 e- → "C" + 2 H2O
|
-0.20 to +0.20
|
It is noteworthy that a manifold of electrochemical processes exist that are able to generate "carbon" on the electrodes' surfaces. We have identified the carbon-material as "graphite-like" by FT-IR spectroscopy (ATR-Bruker Equinox 55). Interestingly, the least reduction potential is required for these processes, which can deactivate the electrodes after approx. 500 DPV-scans. We are able to oxidize the carbon-material at 0.80V (vs. Ag/AgCl). This process is able to regenerate the composite electrode. We are working towards a reductive electrochemical cleaning procedure, which will enhance the lifetime of the composite electrodes without the loss of the carbon-material.
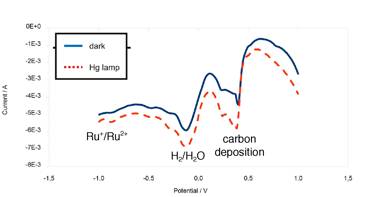
Figure 3: Differential Pulse Voltammograms of the Ru(II)-QP-P3/TiO2 nanogel (sensitizer content: 5.5 percent by weight)/Pd (2.2 percent by weight)/Ag epoxy-electrode. The electrodes were immersed in a 5.0 x 10-2 M NaHCO3 solution, pH=7.2.
It is noteworthy that irradiation increases all three reductive processes: Ru(II) to Ru(I), water to hydrogen and the deposition of carbon. The generation of H2 has been proven by gas-chromatography (Varian 90-P). We are in the process of determining the quantum yields and wavelength dependence of the photoelectrochemical processes.