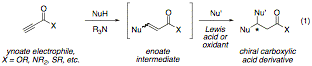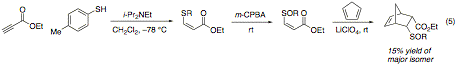Reports: GB1
48322-GB1 Synthesis of Chiral Carboxylic Acid Derivatives via Three-Component Coupling Reactions with Ynoate Electrophiles
Introduction
Our goal is to control the one-pot addition of two disparate nucleophiles to a single ynoate acceptor in order to yield a chiral carboxylic acid derivative (eq 1). The final product of this three-component coupling is a chiral, saturated carboxylic acid derivative. Thus, simple ynoate electrophiles may be converted in one reaction vessel to densely functionalized, stereochemically complex cyclic or polycyclic systems. Successful development of this method represents a valuable advance for a number of reasons: 1) Ynoate esters are readily available, via either carboxylation of acetylene or oxidation of propargyl alcohols. 2) Three-component coupling reactions are attractive because of their efficiency. 3) These reactions rapidly construct stereochemically complex, densely functionalized synthons for target-oriented synthesis.
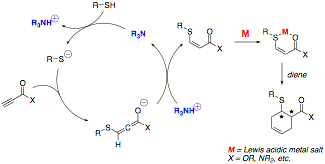
A proposed mechanism for a representative heteroconjugate addition-Diels–Alder process is outlined in Figure 1. First, a trialkylamine base (R3N) deprotonates a thiol nucleophile, generating a thiolate anion and a trialkylammonium cation. Conjugate addition by the thiolate provides an allenolate intermediate, and the allenolate is protonated by the trialkylammonium salt to yield an enoate ester. The enoate ester then acts as a conjugate acceptor; in the example below, it is activated by a Lewis acid (M) and undergoes Diels–Alder cycloaddition. Our ultimate goal is to control the formation of the new stereocenters by the use of a chiral auxiliary or a chiral metal catalyst.
Figure 1. Proposed Mechanism for Three-Component Coupling Reaction of Ynoates
Current Results
We plan a three-pronged approach toward the development of this three-component coupling reaction: 1) establishment of the amine-catalyzed conjugate addition of various nucleophiles to ynoates, 2) use of the conjugate addition products as dienophiles in Diels–Alder cycloadditions, and 3) synthesis of a linked thiol-diene to be used for an intramolecular tandem addition reaction with ynoate electrophiles. To date, the first goal has been accomplished and great progress toward the second goal has been achieved. Goal 3, and development of an asymmetric variant of the reaction, await optimization of our recently established proof of principle (vide infra).
1. Amine-Catalyzed Conjugate Addition to Ynoates
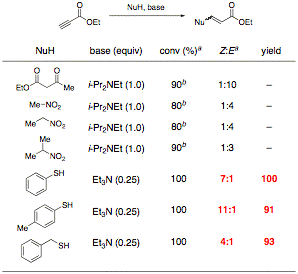
Our investigation of amine-catalyzed conjugate addition to model substrate ethyl propiolate shows promising results, as described in our preliminary proposal (Table 1). In pursuit of our proof of principle, we have chosen to focus on thiol nucleophiles, which require only catalytic amounts of amine and afford excellent yields with high Z:E ratios.
Table 1. Preliminary Results for Amine-Mediated Addition to Ethyl Propiolate
aDetermined by 1H NMR spectroscopy of the unpurified reaction mixture
bAfter workup with aqueous acid
2. Thioether Oxidations
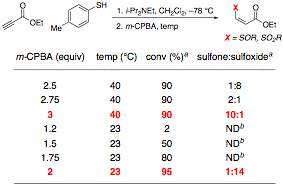
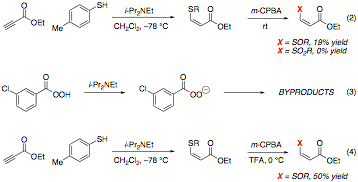
Although production of these thioethers occurs reliably and is easily reproducible, the thioether adducts themselves are not active in Diels–Alder reactions under thermal or Lewis acidic conditions. Accordingly, much of our focus in the last year has concerned the in situ oxidation of these adducts under conditions that are compatible with our initial thioconjugate addition reaction. This compatibility is necessary to guarantee the viability of a one-pot thioconjugate addition-oxidation-Diels–Alder cycloaddition reaction, which is our current goal. Because oxidation to the sulfoxide or sulfone with m-CPBA is compatible with our solvent of choice, CH2Cl2, and provides an electron-poor dienophile, we have pursued the development of a one-pot thioconjugate addition-oxidation sequence that could be expanded to include a Diels–Alder step. After verification that the purified thioether could be oxidized with m-CPBA, conditions for the optimal production of either the sulfoxide or the sulfone were developed (Table 2).
Table 2. Two-Step Thioconjugate Addition-Oxidation Reaction
aDetermined by 1H NMR spectroscopy of the unpurified reaction mixture
bND = Not Determined
As independent steps, both the thioconjugate addition and oxidation steps have been verified to occur in high yield. As a one-pot sequence, however, isolated yields are very poor (<20%) (eq 2). We speculated that interaction between residual trialkylamine from the thioconjugate addition step and the m-CPBA might produce a nucleophilic perbenzoate ion that could interfere with our desired reactivity (eq 3). Addition of trifluoroacetic acid (TFA) to the reaction mixture following thioconjugate addition and oxidation at 0 °C appear to greatly improve the reaction, providing an isolated 50% yield of the purified Z sulfoxide (eq 4). Further optimization and application to our three-step sequence is ongoing.
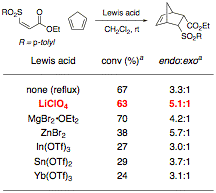
2. Diels–Alder Cycloaddition Reactions
Our preliminary efforts toward developing the
Diels–Alder portion of our reaction focused upon the purified sulfone
oxidation product. A survey of
Lewis acid catalysts at various temperatures showed that LiClO4
appears to be the optimal catalyst for cycloaddition of cyclopentadiene,
providing the product in high conversion and high endo:exo ratio (Table
3). Fortunately, LiClO4
is compatible with the reaction conditions for the thioconjugate addition and
oxidation reactions, and also catalyzes Diels–Alder reactions of
sulfoxides, which we have recently determined to be more convenient dienophiles
than the sulfones under our one-pot conditions.
Table 3. Cycloaddition of Cyclopentadiene to Z Sulfone
aDetermined by 1H NMR spectroscopy of the unpurified reaction mixture
We now report that proof of principle has been achieved for our three-step, one-pot thioconjugate addition-oxidation-Diels–Alder cycloaddition reaction (eq 5). In a single reaction flask, ethyl propiolate was treated with i-Pr2NEt and p-toluenethiol to produce the thioether at ‑78 °C in CH2Cl2. After warming the reaction mixture to room temperature, m-CPBA, LiClO4, and cyclopentadiene were added. After 24 h, 15% yield of the major Diels–Alder adduct illustrated below was isolated, and the structure was confirmed by NOESY. Because the two-step thioconjugate addition-oxidation sequence provides only 19% yield under similar conditions, the Diels–Alder step itself is quite efficient.
Conclusion
We have provided proof of principle for our three-step one-pot reaction sequence, and we have determined that loss in yield occurs during the oxidation step. Our current efforts toward optimizing the two-step thioconjugate addition-oxidation sequence have already raised the yield of the major isomer from 19% to 50%, and we expect a similar rise in yield for the three-step process. Optimization continues, and will be followed by expansion of our sequence to include other thiols and dienes in the near future. Our long-term goals include the development of a tethered thiol-diene bisnucleophile and asymmetric versions of our reaction.