Reports: AC10
47787-AC10 Zeolite Encapsulated Poly(phosphaalkynes): A New Approach to an Elusive Target
The first year of this project has been extremely successful and excellent progress has been made towards its proposed goals. Moreover, several closely related areas of chemistry have emerged from the project, thereby enhancing its scientific impact. The original aim was to initiate a completely new and fundamentally interesting area of chemistry, namely the linear polymerization of phosphaalkynes within the confines of zeolite hosts. The encapsulation of polyphosphaalkynes within zeolite frameworks was expected to be stabilize them towards intra- and intermolecular cycloaddition reactions. As the materials are phosphorus substituted analogues of hugely important polyacetylenes, it was expected they would have unusual opto-electronic and other physical properties. These were to be characterized and the application of polyphosphaalknes as molecular wires, charge storage devices etc. was to be examined, with the eventual aim of developing novel light weight charge storage devices.
Although the project draws on the PI's (Jones) experience in low coordination Group 15 chemistry, the encapsulation of conjugated polymers in zeolites (and all encapsulation technology) represents a completely new field for his group. In addition, Jones has no past experience of any note with the chemistry of "non-molecular" materials, or their exploitation. This project sees an expansion of the topical field of "modern main group chemistry" into the broader realm of materials chemistry, and is now having a significant impact on the PI's overall research portfolio.
Until recently, the sterically unhindered phosphaalkynes (PCR, R = H, Me, Et etc.) to be used in the preparation of the proposed polymers were very difficult to prepare and were thought unstable at all but very low temperatures. In 2001, Guillemin's group showed that, in fact, these phosphaalkynes can be stable for many days at room temperature and are relatively easy to handle. We have made significant modifications to the dehydrochlorination synthetic route they reported and can now routinely prepare batches of, for example, PCMe in multi-gram quantities and of very high purity. As this is the phosphorus analogue of propyne, and the latter has been polymerized within the channels of various zeolites, we have begun to examine its similar polymerization. In the first instance, mordenite and zeolites Y and B (both Na+ and Ni2+ exchanged), have been vacuum dried at 300 oC and exposed to atmospheres of PCMe for periods of 24 h. It appears that, once incorporated in the zeolite pores, the phosphaalkyne quite readily diffuses from the zeolite in the absence of a phosphaalkyne vapor pressure. This has so far hampered efforts to carry out TGA analyses of the samples and spectroscopically characterize them. Mordenite has been exposed to the more reactive parent phosphaalkyne, PCH, though data obtained on the exposed materials strongly suggest decomposition of the phosphaalkyne at the zeolite surface. PCMe appears to have only a loose interaction with the larger pores of mesoporous MCM-41 and is readily rapidly lost from this material in the absence of a phosphaalkyne atmosphere. Similar results have been obtained with the bulkier phosphaalkyne, PCBut.
As the most promising materials appear to be PCMe loaded into Na+ and Ni2+ exchanged mordenite, initial polymerization studies have begun on them. Once loaded, the zeolites have been heated to 100 oC or irradiated with UV light (lambda 254 nm) in sealed vessels under atmospheres of the phosphaalkyne. In all cases, the materials take on a deep orange coloration. Upon isolation, it was found the samples had increased in weight by ca. 4-5%, suggesting significant filling (> 30%) of their pores. Combustion analyses of these materials strongly suggest the weight gains are derived from the initially included phosphaalkyne. Solid state 31P NMR spectroscopy on all materials gave similar results and revealed broad peaks in the region 190-240 ppm, suggestive of the presence of P=C bonds in the included material. There were also broad peaks at ca. 20-50 ppm, probably arising from saturated phosphorus centers, but this region integrated to only ca. 15% that of the lower field region. These initial results provide good evidence for the formation of linear phosphaalkyne oligomers/polymers within the zeolite host, but with the presence of some saturated cycloaddition products. Efforts in the coming 12 months will concentrate on the improvement of polyphosphaalkyne loading levels, obtaining saturated P-center free materials, fully characterizing the materials, and investigating their opto-electronic properties and charge storage capacities.
A related arm of this project has now begun, aimed at examining the incorporation and polymerization of PCMe within the pores of Metal Organic Frameworks (MOFs). MOFs can be considered as analogues of zeolites but have the advantage that they can be obtained as single crystalline materials. As a result, we are investigating MOFs that incorporate acetonitrile, the N-analogue of PCMe, or similar sized molecules. The desorption of the solvent from a number of MOFs, e.g. [{Cd(bpo)(SCN)}.NCMe] and [{Cu3(BTC)2}.(H2O)3], without the loss of crystallinity of the material should allow resorption with PCMe. It is the hope that such phosphaalkyne or polyphosphaalkyne incorporated MOFs will be candidates for single crystal diffractomtery, thereby providing definitive proof for the presence of {-P=C(Me)-} polymers. To this end we have begun a collaboration with an international expert in the field of MOF chemistry, Prof. Kepert (University of Sydney).
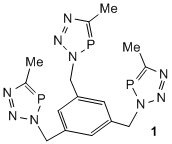
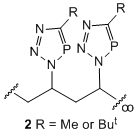
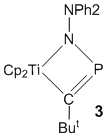
In other "spin-off" arms of this project we are examining the [3+2] cycloaddition of phosphaalkynes with organoazide materials. Although analogous to celebrated "click" addition of alkynes to azides, we have found that reactions with phosphaalkynes are spontaneous and require no catalyst. This has allowed us access to a variety of polydentate and polymeric phosphatriazole materials, e.g. 1 and 2. Polymers 2 are azide free, have MWs of 1800-2300 and narrow polydispersities, PDIs of ca. 1.8-2.0. The coordination and other chemistry of these are being examined in the project. In related work, and in collaboration with Prof. Philip Mountford (Oxford University), we are exploring the use of group 4 metallocene based materials as catalysts for the polymerization/oligomerization of phosphaalkynes. This has, so far, led to the first metallohydrazide/phosphaalkyne cycloaddition product, 3.