Reports: G10
47863-G10 Protonic-Electronic Mixed-Conducting Nanofibers for Energy Conversion
The objective of this project is to use electrospinning to create a novel type of protonic-electronic mixed-conducting nanofiber electrodes for sustainable energy conversion. This ACS PRF fund provides financial support for the PI to establish an active research group to carry out research on developing these novel mixed-conducting nanofiber fuel cell electrodes. The fund is supporting 50% of a graduate student and 10% of a post-doctoral research associates. Both of them get excellent training in materials and fuel cell research, which can help the U.S. stay in a lead position in these strategic fields. The work supported by this fund has scientific and economic impacts. Foundation for fabricating multicomponent nanofibers with controllable nanostructures and functionalities will be established. Research of fuel cell technologies, which will improve air quality, reduce greenhouse gas emissions, and promote alternative energy usage, will also be advanced. In addition to fuel cells, the processing-structure-performance relationships obtained in this work can be used to guide the design of advanced mixed-conducting nanofiber materials for many other applications including batteries, solar cells, biocatalysts, water deionization, electrochromic displays, and protonic and electronic microdevices.
Fuel cells are considered promising candidates for generating power in a clean manner because they provide electricity without combustion and the pollutants associated with burning fossil fuels. The operating principal for fuel cells involves the oxidation of hydrogen and the reduction of oxygen over precious metal catalysts in two electrodes (i.e., anode and cathode):
Anode: H2 à 2H+ + 2e-
Cathode: ½O2 + 2H+ + 2e- à H2O
Overall Reaction: H2 + ½O2 à H2O
A significant barrier to widespread commercial use of fuel cells is, therefore, the cost of these precious metal catalysts (e.g., Pt). To effectively use expensive catalyst, Pt in electrodes must have simultaneous access to reactants, protons, and electrons. In the first year of the project, we synthesize carbon nanofiber-supported Pt, in which the Pt catalyst has simultaneous access to reactants, electrons, and protons, thereby resulting in high catalyst utilization and effective power generation. Carbon nanofibers were synthesized by combining the electrospinning and carbonization techniques. Pt/carbon composite nanofibers were prepared by using two different approaches, i.e., electrodeposition and chemical deposition, respectively.
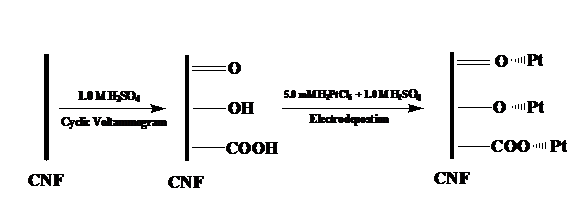
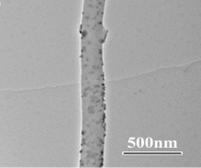
In the first approach, Pt/carbon nanofibers were synthesized by electrodepositing Pt nanoparticles directly onto electrospun carbon nanofibers. Figures 1 and 2 show the procedure of electrodepositing Pt nanoparticles onto carbon nanofibers and the resultant Pt/carbon nanofibers, respectively. The morphology and size of Pt nanoparticles were controlled by the electrodeposition time. The resulting Pt/carbon composite nanofibers were characterized by running cyclic voltammograms in 0.20 M H2SO4 and 5.0 mM K4[Fe(CN)6] + 0.10 M KCl solutions, respectively. The electrocatalytic activities of Pt/carbon composite nanofibers were measured by the oxidation of methanol. Results show that Pt/carbon composite nanofibers possess the properties of high active surface area and fast electron transfer rate, which lead to a good performance towards the electrocatalytic oxidation of methanol. It is also found that the Pt/carbon nanofiber electrode with a Pt loading of 0.170 mg cm-2 has the highest activity.
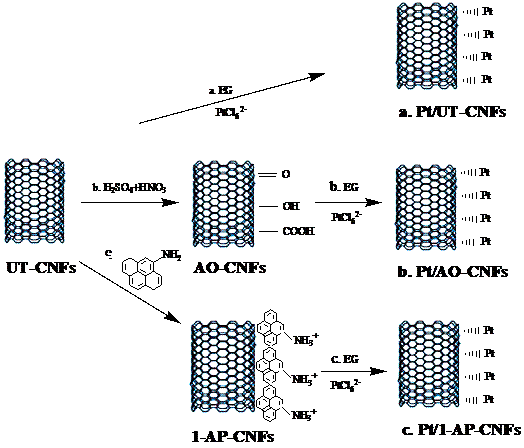
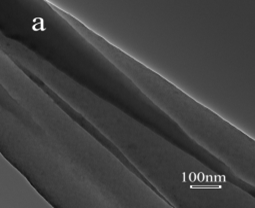
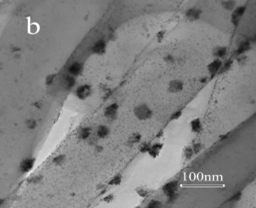
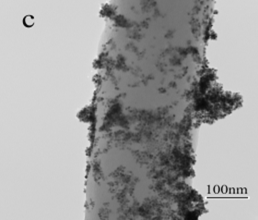
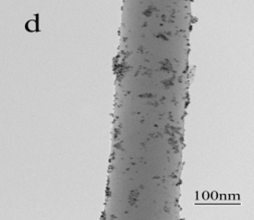
In the second approach, Pt/carbon composite nanofibers were prepared by chemically depositing Pt nanoparticles directly onto electrospun carbon nanofibers using a polyol processing technique. The morphology and size of Pt nanoparticles were controlled by pre-treating carbon nanofibers using acid oxidation or 1-aminopyrene functionalization. Figures 3 and 4 show the procedures of chemically depositing Pt nanoparticles onto carbon nanofibers pre-treated with different methods and their resultant Pt/carbon nanofibers, respectively. It is seen that the pre-treatment using 1-aminopyrene can lead to Pt/carbon nanofibers with smallest Pt particle size and good particle distribution. The noncovalent functionalization of carbon nanofibers by 1-aminopyrene is simple and can be carried out at ambient temperature without damaging the integrity and electronic structure of carbon nanofibers. The resultant Pt/carbon composite nanofibers were characterized by running cyclic voltammogram in 0.5 M H2SO4 and 0.125 M CH3OH + 0.2 M H2SO4 solutions, respectively. Results show that Pt/1-aminopyrene functionalized carbon nanofibers possess the properties of high active surface area and improved performance towards the electrocatalytic oxidation of methanol.
In conclusion, Pt/carbon nanofibers prepared by both electrodeposition and chemical deposition approaches are good catalysts toward the oxidation of methanol, and they can be directly used as electrodes in fuel cells. In Year 2, investigations into the catalytic ability of such composites in MEAs in direct methanol fuel cells will be carried out.
Figure 1. Schematic diagram illustrating the procedure of electrodepositing Pt nanoparticles onto carbon nanofibers.
Figure 2. TEM images of Pt/carbon nanofibers prepared using the electrodeposition method.
Figure 3. Schematic diagram of the synthesis of Pt/untreated carbon nanofibers, Pt/acid-treated carbon nanofibers (b), and Pt/1-aminopyrene-treated carbon nanofibers (c).
Figure 4. TEM images of untreated carbon nanofibers (a), Pt/untreated carbon nanofibers (b), Pt/acid-treated carbon nanofibers (c), and Pt/1-aminopyrene-treated carbon nanofibers (d).