Reports: AC1
45984-AC1 New Low Valent Sulfur and Selenium Acid Derivatives: Reactivity and Synthetic Applications
Sulfenic acid anions are increasingly being recognized as valuable entities for the construction of sulfur containing organic compounds. These compounds are of interest because of the growing recognition for their role in biological systems, because several aspects of their alkylation chemistry remain unexplored and since preparing sulfinyl compounds from sulfenates is counter to the paradigms of sulfur oxidation and nucleophilic attack at sulfur. Recent work by Madec1 clearly shows sulfenate value for the formation of enantioenriched aryl sulfoxides. Any synthetic protocol for sulfenates must work within the limitations of their stability. Specifically, sulfenates are generally not isolable, but must be created from the appropriate precursor and then reacted in the same vessel. One of the viable methods for accessing sulfenates involves their release from beta-sulfinyl acrylates. In our group, this is achieved by treating the beta-sulfinyl acrylates with cyclohexanethiolate to effect a stereospecific addition-elimination reaction, releasing sulfenate anion.2 The beta-sulfinyl acrylates are generally prepared from a conjugate addition/oxidation sequence. However we have developed a protocol for selected E-beta-sulfinyl acrylates by utilizing a new reagent, cesium 2-Z-carbomethoxyethenethiolate.3
This strategy is most applicable
for targeted sulfenates that are base sensitive. As such we have been
investigating the chemistry of a protected cysteinesulfenate
derivative (2). Our addition
elimination chemistry proceeds with minimal perturbation of the sensitive a-proton that normally makes amino acid
chemistry difficult. Under this plan, cysteine
derivative 1 was required for cysteinesulfenate
liberation. During the first period of PRF funding, two different syntheses of 1
were fully evaluated. Analogs of 1 have previously been prepared by The sulfenate (2) was then
generated via thiolate treatment and captured with a
selection of electrophiles including several substituted benzyl bromides
affording diastereomeric sulfoxides (3, Scheme 1). All possible products
were prepared by conventional means and these compounds were used as
identification and calibration standards. Chiral HPLC
analysis was used to establish the diastereomeric
ratio. Yields ranged from 52-99% and diastereomeric
ratios range from 89:11 to 95:5 (14 examples), with the R-configuration
assigned to the sulfinyl group.5 In the burgeoning field of sulfenate chemistry these diastereoselectivities are quite good. Compounds 4 and 5, protected analogs of naturally occurring constituents of garlic
were also obtained by this chemistry. Finally, on a one gram scale, the yields
tend to be much higher than recently reported.5
An interesting feature of the cysteine sulfenate chemistry is the stereochemistry of
alkylation in the presence of 12-crown-4 (12-c-4), a crown ether well known for
its affinity for lithium cations. The introduction of
12-c-4 mildly attenuates the observed dr values,
indicating a) that lithium is important to the stereoinduction
process and b) how strongly the cysteinesulfenate
holds the lithium. The latter is particularly intriguing since 12-c-4 is well
known for its strength to coordinate lithium, but sulfenate 2 appears to be competitive or even
stronger.
It has been proposed that the benzylic sulfoxides diastereoselectively formed by this sulfenate chemistry are
good candidates for intramolecular cyclization
chemistry targeting 7- or 8-membered sulfur/nitrogen heterocycles.
A number of reactions of 3 (X = o-Br) have been evaluated,
but the substrate decomposes under the thermal activation required for the
cyclization. Acid mediated removal of the Boc group
was achieved without perturbation of the sulfenyl
group, but cyclization attempts of those substrates did not proceed. Reduced
forms of compounds 3 are under
investigation.
Marcus Verdu,
an MSc candidate since Jan. 2007, Dr. Suneel P. Singh,
a pdf since late March 2008 have performed the chemistry outlined above. Marcus
has completed his MSc while garnering substantial instrumental skills (NMR,
HPLC, ReactIR) and project
management skills. He has secured employment as an analytical chemist at Testmark Laboratories, a highly accredited, ISO/IEC 17025 certified privately own analysis
company. Dr. Singh will be continuing his appointment, working on
cyclizations of some substrates herein, but also moving to phospholipid
and amino acid synthesis under other funding.
Under this PRF grant we have also
been probing the stereoinduction of alkylation using chiral electrophiles. Specifically,
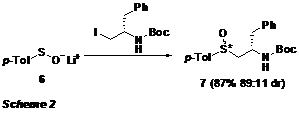
the reaction of sulfenate 6 and an
iodide derived from phenylalanine has been tested as a model system.
Optimization studies are almost complete, with yields reaching 87% with a good dr of 89:11 observed (Scheme 2). Several other sulfenates and electrophiles are
due to be evaluated. This chemistry is ongoing as a major component of the
research of PhD candidate Stefan Soderman who has
received a graduate scholarship through the Canadian funding agency NSERC.
Finally for Adrian Schwan, this funding has permitted a deeper analysis of
sulfenate chemistry from which have arisen a number of new directions. Many of
the ideas will form the basis of other grant proposals for funding from other
sources.
References Cited
1. Maitro, G.; Vogel, S.; Sadaoui,
M.; Prestat, G.; Madec, D.;
Poli, G. Org. Lett. 2007, 9, 5493.
2. O'Donnell, J.S.; Schwan, A.L. Tetrahedron Lett. 2003, 44, 6293.
3. O'Donnell,
J. S.; Singh, S. P.; Metcalf, T. A.; Schwan, A. L. Eur. J. Org. Chem. 2009, 547.
4. Aversa, M.C.; Barattucci, A.; Bonaccorsi, P.; Giannetto, P. J. Org. Chem. 2005, 70,1986.
5. Schwan, A.L.; Verdu, M.J.; Singh, S.P.; O'Donnell j.S.; Ahmadi A.N. J. Org. Chem., 2009,
74, 6851.