Reports: B3
46716-B3 Synthesis, Coordination Chemistry, and Catalytic Studies of a Library of Water-Soluble Polydentate Phosphines Derived from 1,3,5-Triaza-7-phosphaadamantane (PTA)
Bis-phosphines have played an important role in the development of stereochemically controlled catalysis over the past 50 years. Unfortunately, many of the P,P systems are only soluble in environmentally hazardous solvents, and with the greening of the chemical industry, there has been a push to develop chelating and bridging ligands that are soluble in aqueous media. Therefore, we recently undertook the challenge of adding to the library of water-soluble, bis-phosphines by preparing the first ligands containing two PTA (1,3,5-triaza-7-phosphaadamantane) moieties. PTA was chosen as an appropriate synthon because the monodentate, parent ligand is highly water-soluble (235 mg/mL), easy to synthesize, resistant to oxidation, and air-stable. As previously reported, the bis-phosphines, 1,1'-[1,3-phenylenebis(methylene)]bis-3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.1]decane dibromide (1), 1,1'-[1,3-tolylenebis(methylene)]bis-3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.1]decane dibromide (2), 1,1'-[1,3-anisolylenebis(methylene)]bis-3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.1]decane dibromide (3) have been easily prepared in high yields by reacting two equivalents of PTA with 1,3-bis(bromomethyl)benzene, 1,3-bis(bromomethyl)-5-methyl-benzene, 1,3-bis(bromomethyl)-5-methoxy-benzene in dry acetone.
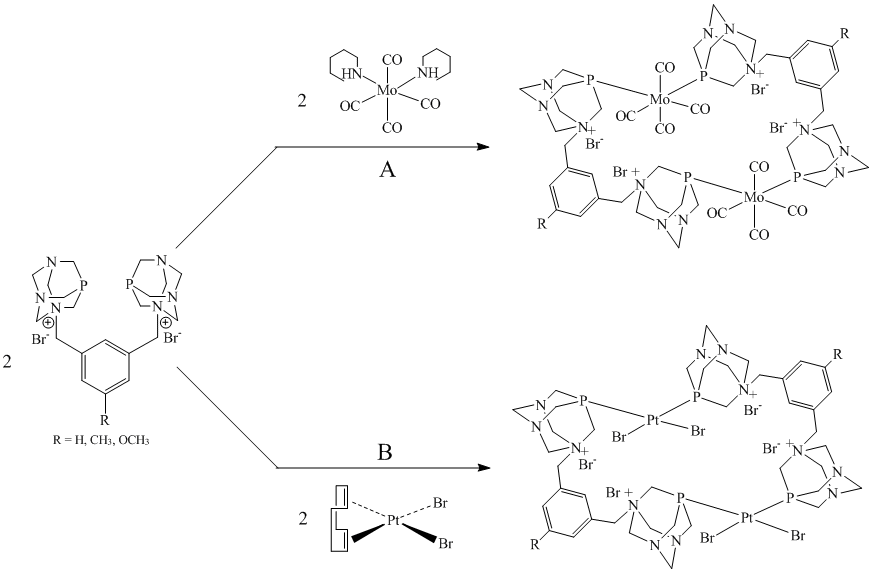
In an effort to explore the coordination chemistry of the bis-phosphines 1-3, the ligands were allowed to react with cis-[Mo(CO)4(pip)2] (pip = piperidine Scheme 1A).
Scheme 1. Synthesis of binuclear metal complexes bridged by bis-PTA xylenes.
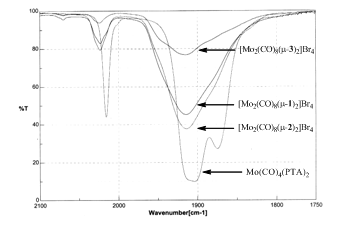
The resulting binuclear complexes cis-[Mo(CO)4(m-P,P)2Mo(CO)4]Br4 (P,P = 1-3) were characterized by NMR, ESI-MS, and IR. The IR spectra (Figure 1) were particularly informative as the CO stretching frequencies allowed the electronic nature of the three P,P ligands to be compared with one another and PTA. As Figure 1 indicates, changing the R group of the phenyl rings had little or no impact on the basicity of the bis-phosphines. The spectra also indicate that the bis-phosphines are less basic than PTA. This is likely due to the fact that 1-3 contain a quaternary N only two bond lengths away from the P. This cationic center draws electron density away from the phosphorous atom thereby making 1-3 poorer Lewis bases than the neutral, monodentate parent.
Figure 1. IR spectra of cis-[Mo(CO)4(m-P,P)2Mo(CO)4]Br4 (P,P = 1-3) and cis-Mo(CO)4(PTA)2.
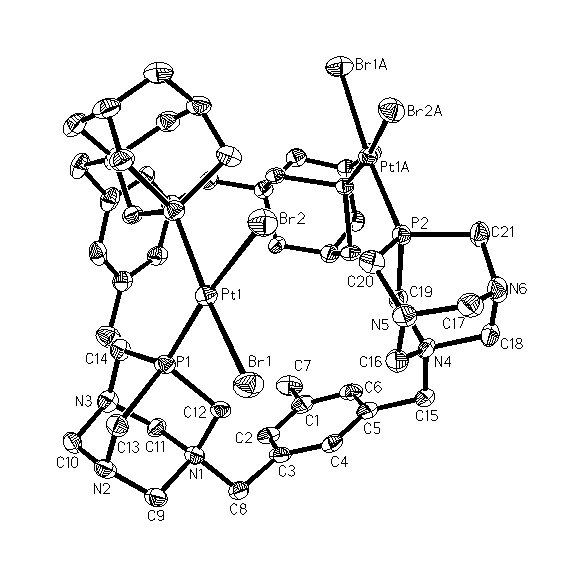
In order to further explore the coordinating abilities of 1-3, the bis-phosphines were combined with cis-[PtBr2(COD)] (COD = 1,5-cyclooctadiene, Scheme 1B). Multinuclear NMR and ESI-MS indicated that 1-3 acted as bridging ligands to form the binuclear complexes cis,cis-[PtBr2(m-P,P)2PtBr2]Br4 (P,P = 1-3). The 31P NMR of these compounds exhibited a series of doublets with 195Pt satellites. The 1JPt-P coupling constants of the three compounds were all approximately 3500 Hz thus indicating a cis P,P geometry about the Pt centers. The fact that the coupling constants were all comparable also implied that the R group of the phenyl backbone had little impact on the basicity of the P,P ligands. This confirmed the findings of the Mo coordination studies. The multiplicity of the spectra indicated that the P atoms were inequivalent on the NMR timescale and hence that the bridging scaffold was quite rigid upon coordination. The bridging nature of the bis-phosphines was further confirmed as cis,cis-[PtBr2(m-2)2PtBr2]Br4 was additionally characterized via single crystal X-ray diffraction (Figure 2). As expected, the geometry about both Pt atoms was square planar. The P-Pt-P bond angle was greater than 90o about each Pt center thus further validating the belief that the phenyl backbone was constrained. This steric strain is likely why 1-3 preferentially act as bridging and not chelating ligands.
Figure 2. Thermal ellipsoid representations of cis,cis-[PtBr2(m-2)2PtBr2]Br4. The bromide anions and solvate molecules have been omitted for clarity.
The coordination studies collectively illustrated that alternation of the R group on the phenyl backbone of the P,P systems did not alter the ligand's basicity and that their basicity is less than that of the parent PTA. More importantly, it also showed that the bis-PTA compounds effectively and preferentially function as bridging ligands. This may prove to be quite valuable as multinuclear metal complexes often exhibit enhanced catalytic activity. In addition to proving that these bis-PTA ligands have great promise for future fruitful study, this research has also been very important because it impacted the lives of the undergraduates involved. Two students will graduate in the spring of 2010, and both plan to attend graduate school to study either inorganic chemistry or material science. One student commented at the end of the summer that this experience confirmed his desire to become a professional, synthetic chemist. In short, the initial work with bis-PTA ligands has allowed several young scientists to experience the thrill of discovery while advancing the ever important field of green chemistry.