Reports: G1
46278-G1 Development of C-H Insertion on Sulfonyl Compounds
In the second year of the funding period
the previously initiated projects were further developed, contributing to the
established program in the area of the organic synthesis. 3 graduate and 11
undergraduate students were involved in research. The PI started participating
in programs to involve high school students (Pilot-to-Lab) and Native
American students (NATURE programs) in research. 8 presentations on international
conferences were made, 4 peer reviewed articles were published acknowledging
the support of the ACS PRF grant.
Three projects were performed: 1)
development of C-H insertion on sulfonyl compounds as a synthetic method 2)
synthesis of Plakortethers, and 3) approach to the synthesis of Terpiodiene.
The previously discovered C-H insertion
on sulfonyl compound was explored in the direction of its synthetic application.
We've
previously started the study of the alkylation of the C-H inserted products. We
went on to discover that the high stereoselectivity, observed in alkylation of
the six-membered products also is observed in the alkylation of the
five-membered cyclic products. The stereochemistry of the alkylation products
was determined by NOE correlations. Chlorination using C2Cl6
was also possible. It was additionally found that the alkylation products can
be rearranged by treatment with NaHMDS, and converted to promising cyclopentene
intermediates, such as 1.1, by Ramberg-Baecklund
reaction (Scheme 1).
Scheme 1
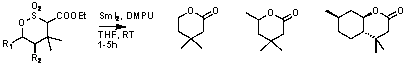
Experimentation on sulfonates revealed
that reductive scission of the C-S bond was possible using SmI2/DMPU
at prolonged reaction times (Scheme 2).
Scheme
2
Then we proceeded by application of the
reaction in synthesis. The first synthetic target was selected to be
hepatoprotective antibiotic Bakuchiol. The challenging element in its structure
is the quaternary chiral center. Using the developed methodology it could be
created from a tertiary center of a commercially available (-)-citronellol,
using the sulfonate modification to effectively control regioselectivity.
Using the developed sequence, sulfonate 3.1 was prepared from citronellol
(Scheme 3). The initially planned SmI2 desulfonation was,
unexpectedly, quite ineffective, causing formation of multiple byproducts.
Alkylation of 3.1 was possible.
However, this direction required decarboxylation that was not expected to be
easy. Meanwhile, it was found that DIBALH reduction would provide alcohol 3.2 in high yield. This alcohol could
be converted to alcohol 3.4 by treatment
with Ph3P/NBS, and then Zn in DME, albeit in low yield. An
alternative high-yielding method was found via elimination and desulfonation of
the resulting vinylic sulfonate, 3.3.
Of the reagents attempted (Li/NH3, BuMgCl-Ni(acac)2,
BuMgCl-Ni(acac)2, SmI2, SmI2/DMPU,
Na-EtOH-THF) the best results were obtained using Bu3SnLi. The
resulting alcohol, after oxidation, was converted to Bakuchiol as previously
described. Mosher ester study also confirmed that no loss of optical purity
took place.
Scheme
3
The second project was the synthesis of
Plakortethers secondary metabolites from marine sponge Plakortis Simplex (Scheme 4). We previously developed a sequence
for the synthesis of the key intermediate, 4.1,
via taking advantage of the symmetry in the molecule. We went on to optimize
the aldol condensation step. Additionally, on closer analysis it was revealed
that the 13C spectrum of the prepared Plakortether G has several
deviations from the reported values. To conclusively prove the structure,
additional studies were performed. NOE correlations confirmed relative
configuration around the tetrahydrofuran ring. Confirmation of the side chain
configuration, however, was not available. Several attempts to obtain crystalline
derivatives for X-Ray analysis were unsuccessful. Finally, it was learned that
both Plakortethers F and G, upon treatment with pyridinium tosylate, convert to
the same bicyclic product, 4.2. This
confirmed the identical configurations of all the centers in both compounds
outside the acetal center. The NOE study of the cyclized product confirmed
configuration of the centers in the side chain. After communication with the
authors of the original isolation paper, we were provided with a copy of the 1H
spectrum of Plakortether G, which was a nearly photographical match to our
compound. This lead us to believe the two compounds were identical, despite the
13C spectrum differences.
Scheme 4
Synthesis of other Plakortethers as well
as Simplakidine A is now being performed. Additionally, an agreement has been
achieved on a collaborative project involving biological testing of
Plakortethers, synthetic intermediates and possible analogs to explore and
optimize their biological activity.
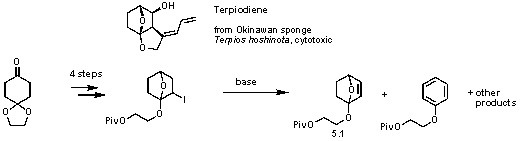
Finally, studies on the synthesis of Terpiodiene,
an isolate from an Okinawan sponge Terpios
hoshinota with cytotoxic activity, were continued.
Scheme 5
The previously discovered route for
preparation of alkene 5.1 provided
very low yields. Attempts to optimize it (via a change of solvent to THF, DMF,
DMSO) and base (K2CO3, KOt-Bu, LDA) were not successful.
The constant complication kept being overelimination, leading to aromatization
(Scheme 5). Attempts to use sulfo and selenocyclization instead of
iodocyclization also proved difficult and low yielding.
An alternative route based on
intramolecular aldehyde allylation was concocted (Scheme 6). It is hoped that the
possible competing elimination can be suppressed by a choice of metal and
conditions. So far preparation of the key substrate proved heavily complicated
by an extremely easy aromatization.
Scheme 6
Currently, alternative approaches to
this intermediate using C-H insertion and olefin metathesis are being explored,
as well as a possibility of using other reaction besides the allylation to
construct the same bond (such as radical processes) to avoid the use of
substrates and conditions conducive of elimination and aromatization.
In conclusion, with the support of the
ACS Petroleum Research Fund we were able to establish a research program in the
area of synthetic organic chemistry featuring both development of new synthetic
methods and targeted synthesis of natural products. 4 peer reviewed
publications have been published and 8 international conference presentations
have been made with the results of the ongoing projects. The established
program is well positioned for further advancement and diversification.