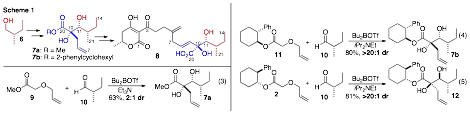Reports: AC1
48199-AC1 New Synthetic Methods Involving Wittig Rearrangement Processes
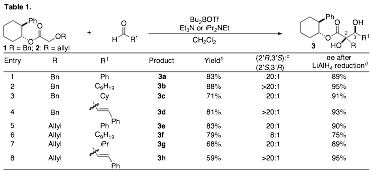
During the past year a significant portion of our research efforts have been directed towards the development of an asymmetric tandem Wittig rearrangement/aldol reaction, as described on pp 19–21 of our original proposal. A brief study on the use of chiral Boron reagents to achieve asymmetric induction failed to provide useful yields and selectivities. However, use of readily available enantiopure alcohols as chiral auxiliaries proved more fruitful. As shown in Table 1, the tandem Wittig rearrangement/aldol reaction between 2-phenylcyclohexyl esters 1 and 2 provided syn-diols 3 in good yield with excellent stereoselectivity.
a Conditions: 1.0 equiv 1 or 2, 1.5–2 equiv R1CHO, 3.2 equiv Bu2BOTf, 4 equiv Et3N (R = Bn) or iPrNEt2 (R = allyl), CH2Cl2, 0°C → rt → 0°C. b Isolated yield (average of two or more experiments). c Ratios were determined by 1H NMR analysis. All products were obtained with >20:1 syn:anti selectivity. d Enantiomeric excess was determined by chiral HPLC or Mosher ester analysis after reduction to the corresponding triol with LiAlH4.
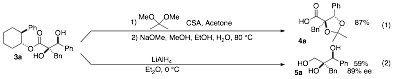
Cleavage of the chiral auxiliary from the product was accomplished through one of two approaches. Conversion of 3a to the corresponding acetonide followed by basic hydrolysis provided carboxylic acid 4a in 87% yield. Alternatively, reductive cleavage of the auxiliary afforded triol 5a in 59% yield and 89% ee.
In order to illustrate the synthetic utility of this transformation we sought to prepare 7b, which is closely related to a key intermediate (7a) in Trost's synthesis of alternaric acid (Scheme 1). Ester 7a was previously generated from commercially available (S)-2-methyl-1-butanol (6) in seven steps (longest linear sequence). The C10–11 diol functionality was introduced via Sharpless asymmetric dihydroxylation (AD) of a trisubstituted enoate, and the pendant terminal alkene was installed in subsequent steps. In principle 7a could be generated through a Wittig rearrangement/aldol reaction sequence between methyl ester 9 and enantiopure aldehyde 10 (prepared in one step from 6). However, addition reactions of nucleophiles to 10 are known to occur with poor diastereoselectivity due to the similar steric properties of the aldehyde C2-substituents (Me vs. Et). As anticipated, the coupling of 9 with 10 proceeded with modest Felkin selectivity to afford 7a with only 2:1 dr (eq 3). In contrast, the (1S,2R)-2-phenylcyclohexyl ester 11 was transformed to 7b in 80% yield as a single stereoisomer (eq 4). Overall our synthesis of 7b requires only three steps in the longest linear sequence, as ester 11 was prepared in two steps from commercially available materials. Importantly, our strategy complements Sharpless AD chemistry, as the Wittig rearrangement/aldol reaction allows for preparation of a,b-dihydroxy esters bearing relatively nucleophilic alkenes that would not tolerate typical dihydroxylation conditions. In addition, the auxiliary provides complete control over relative stereochemistry in this system, as (1R,2S)-ester 2 was converted to 12 in 81% yield with >20:1 dr.
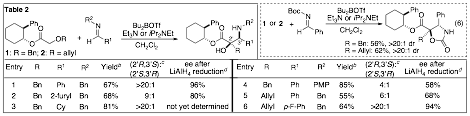
We have also sought to extend the scope of tandem Wittig rearrangement/enolate trapping reactions to allow use of imine electrophiles as outlined on pp 23–24 of our original proposal. We have successfully developed a new tandem asymmetric Wittig rearrangement/Mannich reaction. As shown below (Table 2), a number of amino alcohols are generated in good yield with good to excellent stereoselectivity when N-benzyl or N-aryl imines are employed. Use of N-Boc imines leads to the formation of trisubstituted isoxazolidin-2-ones in moderate yield with excellent stereocontrol (eq 6).
a Conditions: 1.0 equiv 6f or 10, 1.5–2 equiv R1CHO, 3.2 equiv Bu2BOTf, 4 equiv Et3N (R = Bn) or iPrNEt2 (R = allyl), CH2Cl2, 0°C → rt → 0°C. b Isolated yield (average of two or more experiments).
During the coming year we intend to further expand the scope of the tandem Wittig rearrangement/enolate trapping reactions. As outlined in our original proposal, we plan to conduct experiments that will provide insight into the mechanism of 1,2-migration. This knowledge should aid our efforts to broaden the range of groups that undergo migration. In addition, we plan to further explore the range of electrophilic coupling partners that can be use in this chemistry.