Reports: G10
48733-G10 Demonstrate the Feasibility of Using Triplet Polymers with Variable Bandgaps for Efficient Photovoltaics
We have studied the solvent effects on the morphology for polymer/C60 derivative films using chlorobenzene, 1, 2-dichlorobenzene and 1, 3-dichlorobenzene. Atomic force microscopy (AFM) was performed to study the film morphology using chlorobenzene, 1, 2-dichlorobenzene and 1, 3-dichlorobenzene as solvents. Initial studies showed that the size of nanocrystallites in 1, 2-dichlorobenzene based films is smaller than nanoclusters in the other two films. These nano structures should be contributed into the congregation of PCBM. In addition to the film morphology, we also studied the polymer calculation on the polymers to extend its absorption spectra into infrared regions.
Morphology Study
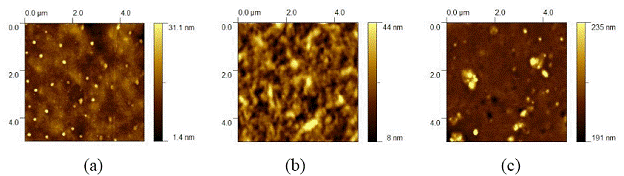
Figure 1 shows 5 µm scale top mode AFM images of the chlorobenzene based films from (a) P3HT/PCBM blend; (b) P3HT only film; (c) PCBM only film, respectively. Nanoclusters (bright dots) are distributed throughout the P3HT/PCBM blend, as shown in Figure 1a. Such clusters were also reported in MDMO-PPV/PCBM blends by others. Images of the P3HT only film do not show such clusters but a typical polymer morphology (Figure 1b). In the PCBM only film, nanoclusters were also found (Figure 1c). These AFM images imply that nanoclusters are from the PCBM aggregation, which is consistent with previous reports on polymer/fullerene blends.
Figure 1. 5 µm scale top mode AFM images of the chlorobenzene films from (a) P3HT/PCBM blend; (b) P3HT only film; (c) PCBM only film.
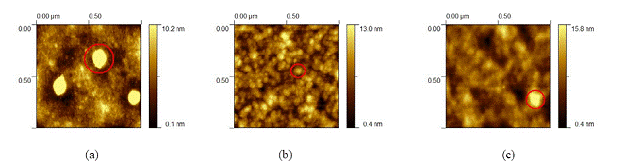
Figure 2: 1 µm scale AFM images of top mode of the P3HT/PCBM blend fabricated via (a) chlorobenzene; (b) 1, 2-dichlorobenzene; (c) 1, 3-dichlorobenzene. (One nanocluster is picked up by one red circle to highlight in each image.)
Figure 2 shows 1 µm scale AFM images of top mode of P3HT/PCBM blends fabricated by chlorobenzene, 1, 2-dichlorobenzene and 1, 3-dichlorobenzene, respectively. From these pictures, it was found that nanoclusters and nanocrystallites appeared in all three samples, which can be contributed to the solubility of PCBM in organic solvents. However, nanocrystallites in 1, 2-dichlorobenzene based films is more dispersive and homogenerous than nanoclusters in the other two films. The average diameter of PCBM nanoclusters in the chlorobenzene based blend was ~ 120 nm, as shown in Figure 2a. The 1, 2-dichlorobenzene based blends showed a homogenerous distribution of many nanocrystallites with average size of ~ 40 nm (Figure 2b). In the 1, 3-dichlorobenzene based blend, the nanoclusters with average size of ~ 80 nm were observed (Figure 2c). These results indicate that 1, 2-dichlorobenzene and 1, 3-dichlorobenzene led to smaller nanoclusters than chlorobenzene, especially in 1, 2-dichlorobenzene based blends.
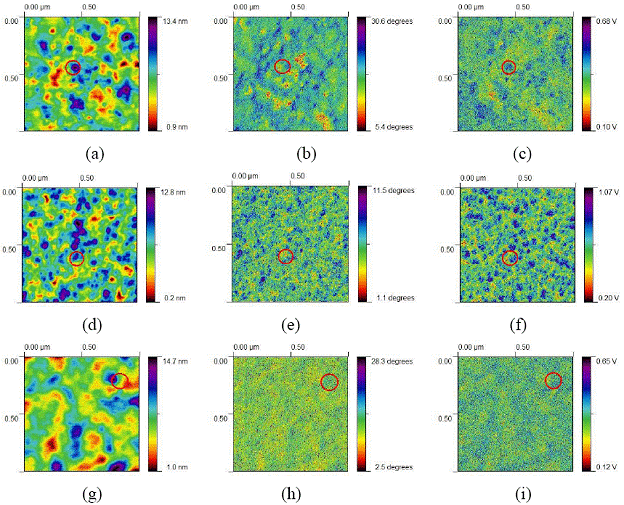
Figure 3: 1 µm scale images of (a) top mode of the P3HT/PCBM blend fabricated via chlorobenzene; (b) phase mode of the blend fabricated via chlorobenzene; (c) surface potential mode (tested by KFM) of the blend fabricated via chlorobenzene. (d) top mode of the P3HT/PCBM blend fabricated via 1, 2-dichlorobenzene; (e) phase mode of the blend fabricated via 1, 2-dichlorobenzene; (f) surface potential mode (tested by KFM) of the blend fabricated via 1, 2-dichlorobenzene; (g) top mode of the P3HT/PCBM blend fabricated via 1, 3-dichlorobenzene; (h) phase mode of the blend fabricated via 1, 3-dichlorobenzene; (i) surface potential mode (tested by KFM) of the blend fabricated via 1, 3-dichlorobenzene.
Figure 3 shows 1 µm scale images of top, phase modes and surface potential image of blends fabricated by chlorobenzene, 1, 2-dichlorobenzene and 1, 3-dichlorobenzene, respectively. The complementary information, which is required to analyze topographic, microscopic phase separation and the electronic quality of the interfaces, are provided collectively by these AFM and KFM images. The phase separation between P3HT and PCBM was found in these topography, phase and surface potential images. It was found that PCBM nanoclusters were surrounded by P3HT with clear edges in the top and phase images. The area marked by red circles indicates the effect of sub-surface donor/acceptor network in surface potential measurements. These areas showed significant deviation between phase and surface potential images indicating charging of submerged PCBM due to the charge transfer between two phases.
Figure 3 also shows the difference among blends from three solvents. Both P3HT and PCBM phases were located in isolated area, which was caused by large PCBM nanoclusters in the chlorobenzene based blend. Homogenerous distribution of phase separation was observed in the 1, 2-dichlorobenzene based blend, in which the PCBM nanoclusters are much smaller, compared with other two blends. In the 1, 3-dichlorobenzene based blend, both phases distributed like chains.
Polymer Calculation
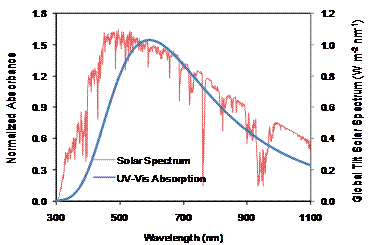
We have calculated our target polymers using density functional theory (DFT) and the results showed a super-broad UV-VIS-NIR absorption spectrum (Figure 4) up to 1050 nm with a band gap of ~ 1.0 - 1.1 eV.
Figure 4: The UV-VIS-NIR absorption spectra showing the polymer has a very efficient light harvesting capability because its absorption spectrum matches the solar spectrum very well. The absorption was obtained from DFT based quantum chemical calculation.
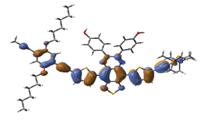
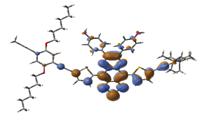
We also studied the planarity of the newly designed polymers via DFT calculation. A high degree of planarity was found in these polymers (Figure 5), which was expected to lead to a high possibility of packing and carrier mobility. In addition, an efficient delocalization throughout the backbone chain was observed in the LUMO orbitlas.
Figure 5: HOMO and LUMO wavefunctions calculated using DFT-B3LYP/LanL2DZ for the quinoxaline based triplet polymer: HOMO (-4.46 eV) and LUMO (-3.40 eV).
Acknowledgement
Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research. We also acknowledge for the work on molecule calculation in collaboration with Dr. Seth Darling at Argonne National Laboratory. Use of the Center for Nanoscale Materials was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. In addition, this work was supported in part by the MRSEC Program of the National Science Foundation under Award Number DMR-0819885.