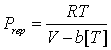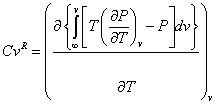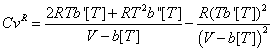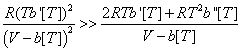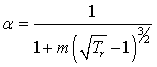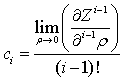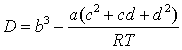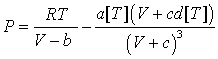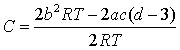Reports: B6
47338-B6 A Novel Theoretically-Based Approach for Developing Equation of State Models
One of the most important projects of the research strategy listed in the original proposal is evaluation of the temperature dependencies for the developed Equation of State (EoS). This equation originates from the hard-sphere approach, which claims the temperature-independent covolume b. However, real molecules do not have hard repulsion potentials. This fact has been considered by several theoretically-based models, including different modifications of SAFT, which have introduced temperature dependencies for their size parameters. In fact, such dependencies may substantially increase the flexibility of EoS and, theoretically, allow a simultaneous fitting of the volumetric properties of real compounds, their vapor pressures, and the virial coefficients. An important issue that should be considered prior further development is the consequences of making the covolume temperature-dependent for the isochoric heat capacities.
The repulsive term with temperature-independent covolume has a zero contribution to the residual isochoric heat capacities. Making the covolume temperature dependent as follows:
and substituting eq 6 into the equation:
yields:
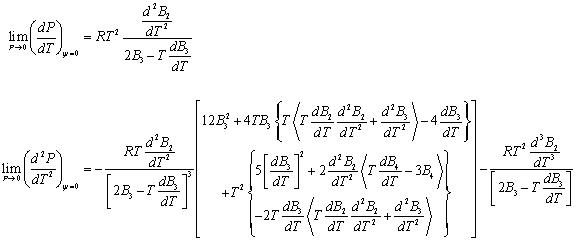
At very high pressures V will approach b[T] and both denominators will become very small numbers. Since, in addition, R(Tb' [T])2 is always positive, it can be seen that:
Hence, at very high pressures Cvr will necessarily become a large negative number. Obviously, at the infinite pressure its value is indeterminate. Such a non-physical result totally excludes the possibility of further consideration of eq 1.
The advanced hard-sphere repulsive terms, such as the one of Carnahan and Starling attached by the temperature-dependent covolume, may not yield the indeterminate residual isochoric heat capacity at the infinite pressure. Nevertheless, the positive values of b'[T] and b''[T] still result in the non-physical negative values of Cvr. At the same time, the negative b'[T] results in another non-physical phenomena, namely, the crossing of isotherms. In addition, the negative b''[T] contradicts the theoretical studies of softly repulsive systems.
These results indicate that not everything justified by the molecular theories may finally become a robust and reliable EoS model. Currently we put major efforts for finding creative solutions to the problem. In particular, the temperature dependent and temperature-independent covolumes can be introduced in the repulsive term.
Another important issue is development of the temperature dependency for the attractive term that will not introduce non-physical breaking points and will improve the predictions of the auxiliary properties. At the first stage of the research we consider the empirical functionalities and later will adjust them to the existing molecular theories. In fact, none of the currently available temperature dependencies has been found appropriate for the aims of our research. After examining a plenty of possible theoretically-based and empirical expressions, a following simple one-parameter temperature dependency that asymptotically approaches zero at high temperatures has been developed:
A very important issue that has not receive an appropriate attention thus far is the inability of most analytical EoS models to describe several experimental facts, namely the decrease of the 3rd virial coefficient with temperature and the maxima established by the isochoric heat capacities near the critical temperatures. Both phenomena are related to each other.
After some algebra the following simple and universal solution can be found for virial coefficients:
where ci is the i-th virial coefficient. Substitution of eq 6 into generalized cubic EoS yields:
where B, C and D are the 2nd, 3rd and 4th virial coefficients. Inspection of eq 8 indicates that the desired behavior of C can be obtained if the sum (c + d) is a small negative number. Regrettably, an accurate description of the volumetric properties and B by cubic EoS requires positive values of (c + d). However it should be pointed out that the situation can be different when considering non-cubic van der Waals-like models. For example, the EoS:
yields the following expression:
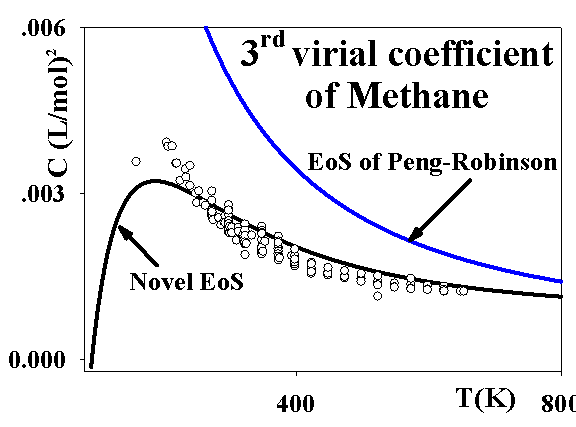
If d > 3, C decreases with temperature and visa versa. This result suggests introducing the temperature-dependent d, which could become bigger or smaller than 3 at different temperatures. The results are presented on the following figure:
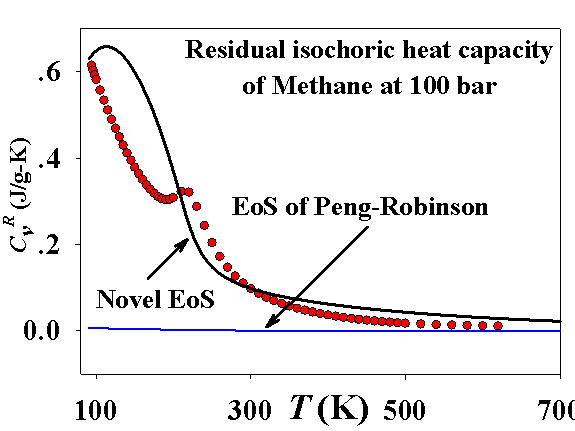
Improving the predictions of the 3rd virial coefficient has a very positive impact on the results for the residual isochoric heat capacities. Remarkably, that the model qualitatively correctly presents a maxima of this property near the maxima of the 3rd virial coefficient, as shown on the following figure:
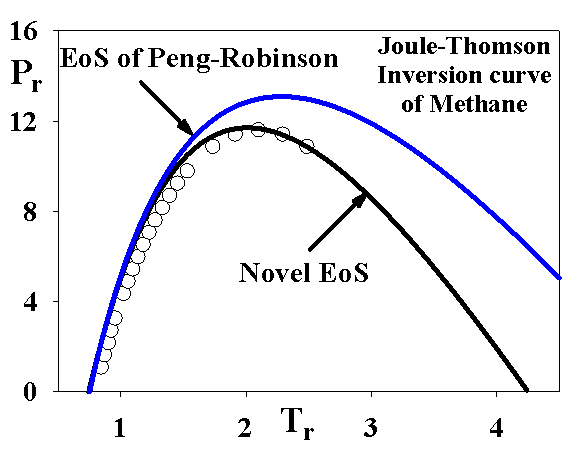
Our research has confirmed the fact that the 3rd virial coefficient is related also to the Joule-Thomson inversion curve and the following relation has been developed:
The following figure demonstrates the improvement achieved by the novel EoS model:
Similar results are obtained also for other compounds. Analysis of empirical functions such as eq 10 could give further directions for development of theoretically-based expressions.
The present research has a very important impact on the principal investigator's career. The support of the Petroleum Research Fund allows to the principal investigator publication in the leading chemical engineering research journals. It is hard to overestimate the impact of the scholarships from the Petroleum Research Fund on careers of the undergraduate students participating the research. This support allows them to dedicate full time to the research work, to earn many important skills, such as the ability to use the professional Data Bases and the software and, finally, to be better engineers. Some of the students have become co-authors of published papers, which could contribute a lot for their chances to be accepted to the post-graduated programs in other institutions in Israel or abroad.