Reports: AC3
47472-AC3 Heterolytic Activation of Hydrogen Promoted by Ruthenium Nanoparticles on Poly(vinylpyridine) and Hydrogenation of Aromatic Compounds
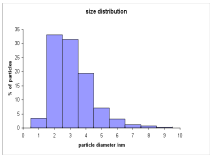
1. Catalyst preparation and characterization. New catalysts composed of Ru nanoparticles immobilized on poly(4-vinylpyridine) (PVPy) were synthesized by reduction of RuCl3.3H2O with NaBH4 in the presence of the polymer and characterized by use of Transmission Electron Microscopy (TEM). A new material containing 10% Ru consists of nanoparticles with a narrow particle size distribution (average size 3.1 nm –Ferret diameter) imbedded in the polymer. After a hydrogenation run, an increase in the average particle size to 5.9 nm was observed.
Fig. 1. TEM image and particle size distribution of 10% Ru/PVPy
We have also applied a similar method to produce a nickel-based catalyst on PVPy with an average particle size of 2.5 nm, which suffers only a very slight increase in particle size (to 3.0 nm) after a hydrogenation run.
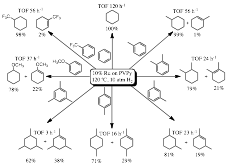
2. Catalytic properties. The new catalysts are efficient for the hydrogenation of aromatic compounds, representative of components of petroleum-derived fuels. The effect of various reaction parameters (T, P, solvent, [substrate]:[catalyst]) were evaluated systematically using toluene as a model substrate. Under the optimized reaction conditions (120 ¼C, 10 atm H2) a series of benzene derivatives could be hydrogenated partially or fully by use of 10% Ru/PVPy (Fig. 2):
Fig. 2. Hydrogenation of benzenoid aromatics by 10% Ru/PVPy
The
hydrogenation rate is decreased by increasing steric congestion, but it is not greatly influenced by the
presence of electron-donating or -withdrawing substituents on the ring. Bicyclic
aromatic hydrocarbons are also hydrogenated albeit with lower rates.
Naphthalnene is specifically reduced to 1,2,3,4-tetrahydronaphthalene (TOF 0.7
h-1) while anthracene is converted to 1,2,3,4-tetrahydroanthracene
(TOF 2.0 h-1).
Nitrogen
containing molecules representative of fuel components may also be hydrogenated
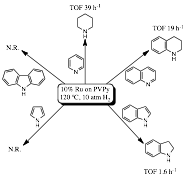
by use of Ru/PVPy under moderate reaction conditions. Pyridine, quinoline and
indole were reduced with good rates exclusively at the heterocycle, while
pyrrole and carbazole were not hydrogenated under the reaction conditions
employed (Fig. 3). Organosulfur compounds of importance of fuel processing,
like thiophene and benzothiophene were not hydrogenated by this catalyst under
the mild reaction conditions employed.
Fig. 3.
Hydrogenation of organonitrogen compounds by 10% Ru/PVPy.
Furthermore,
it was observed that the presence of thiophene inhibited the hydrogenation of
toluene. The recyclability of the catalysts was also demonstrated by using the
same catalyst sample in three consecutive runs of quinoline hydrogenation, with
little variation in the TOF or selectivity between the different cycles. A
paper containing these results is in preparation for publication.
Preliminary
data for the Ni/PVPy catalyst indicates activity for the hydrogenation of
toluene to methylcyclohexane (TOF 6.0 h-1; 100 ¼C ; 40 atm H2)
and of quinoline to 1,2,3,4-tetrahydroquinoline (TOF 0.7 h-1; 100
¼C; 20 atm H2).
3. Hydrogenation mechanism. Our catalyst design involves metallic nanoparticles
intimately associated with a basic support, as a means to promote the
heterolytic activation of H2 (Fig. 4). This is a well-established mechanism
in solution chemistry but it is extremely rare on solid catalysts.
Fig. 4. Heterolytic
activation of H2 on Ru/PVPy.
The
splitting of H2 into H- (on the metal) and H+
(on the pyridine sites) would lead to ionic hydrogenation mechanisms, which
should result in resistance to poisoning. In previous work on the hydrogenation
of quinoline by Ru/PVPy we demonstrated that the rate increased with solvent polarity
and with addition of bases or acids and these effects were taken as indirect
evidence in favor of an ionic hydrogenation mechanism. In order to obtain
direct evidence for the heterolytic splitting of hydrogen, we have attempted to
identify the pyridinium fragments
in the catalysts after treatment with hydrogen gas by FTIR spectroscopy. In
control experiments, the polymer and the Ru/PVPy catalyst were protonated with
several acids, in order to identify diagnostic IR bands of the pyridinium
moeity at XXX and XXX cm-1. Subsequently, the catalyst was treated
with hydrogen gas under pressure and analyzed by FTIR; this analysis did not
reveal the bands associated with the protonated nitrogen. These results may be
interpreted either as evidence that the hydrogen splitting shown in Fig. 4 is
reversible and therefore the protonated form of the catalyst is only stable
under hydrogen pressure, or that the hydrogen addition takes place
homolytically on the metal nanoparticles, without involvement of the support.
This point is being further investigated.
4. Organometallic complexes in
heterolytic hydrogen activation. In
order to provide further mechanistic insights on the hydrogenation mechanisms
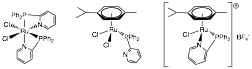
operating in the solid catalysts, we have studied the reactions of H2
with related soluble molecular complexes that can be studied in solution by NMR
methods and may serve as models for the reactions of the Ru/PVPy catalysts:
Although
these compounds activate hydrogen under pressure, as demonstrated by their
ability to hydrogenate quinoline, the active species containing hydrides and/or
protons could not be characterized spectroscopically, most likely due to the
reversible nature of the hydrogen addition, which would make them unstable in
the absence of excess hydrogen under pressure. We are currently extending these
studies to complexes containing pyridyl-N-heterocyclic carbene ligands, which
are expected to be more reactive and lead to more stable products.
The reactions of hydrogen with a different set of Ru
and Rh complexes containing the anionic tripodal phosphine ligand [PhB(CH2PPh2)3]-
were studied.
The
Ru complex [(PhBP3)Ru(m-Cl)]2
was shown to activate hydrogen heterolytically in the presence of external base
(Et3N), while the related Rh derivative [(PhBP3)Rh(cod)] reacted through
oxidative addition to produce a dihydride, even in the presence of external
base. These compounds were not active in the hydrogenation of aromatics but
they displayed a remarkable activity and selectivity for the reduction of the C=O
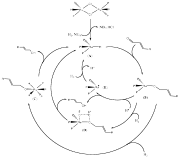
bond of alfa,beta-unsaturated aldehydes. The catalytic cycle for the Ru system
is shown in Fig. 5. These results were
published in Organometallics 2009).
Fig. 5. Catalytic cycle for the
hydrogenation of cinnamaldehyde catalyzed by [(PhBP3)Ru(m-Cl)]2.