Reports: AC7
48228-AC7 Solid Rheology of Glassy Substances: Polymer and Colloidal Systems
Introduction
There is considerable interest in the relationships between colloidal and molecular glass-formers. The present work addresses these via a combination of light scattering from colloids and mechanical measurements on colloids and polymer glasses. The past year has focused primarily on the area of the light scattering measurements from a thermoresponsive particle dispersion in water.
Background
For molecular glasses,
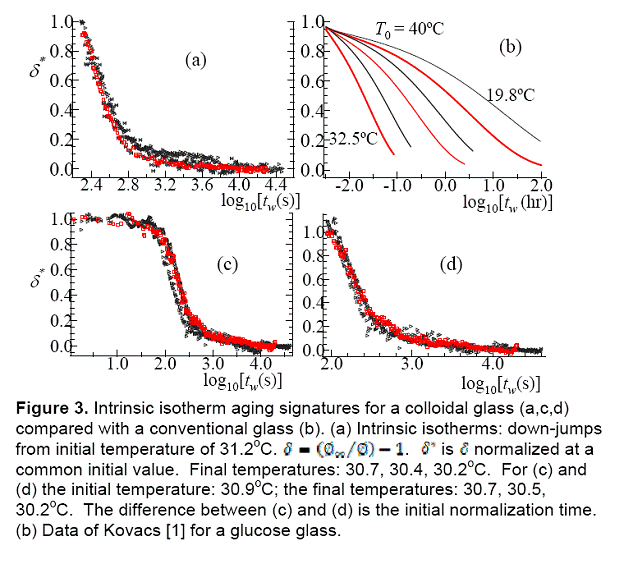
Kovacs [1] catalogued three aging signatures: (a) intrinsic isotherms (b) asymmetry
of approach, and (c) memory. Intrinsic isotherms involve temperature jumps
from Tg to a number of temperatures below Tg.
The set of curves for a thermodynamic quantity as a function of aging time tw
is called the intrinsic isotherms. Asymmetry of approach involves comparison
of up- and down- temperature jumps: (a) first equilibrating the sample at Tf
+ ![]() and jumping to Tf; (b) repeating
(a) with Tf -
and jumping to Tf; (b) repeating
(a) with Tf - ![]() . It is found that the profile of V vs. tw
is asymmetric between two T-jumps +
. It is found that the profile of V vs. tw
is asymmetric between two T-jumps +
![]() and -
and - ![]() although it would be symmetric were the response linear.
For the memory experiment, the sample is partially aged at a low temperature Ti
after which T is increased to T=Tf<Tg and
the volume response is measured. Under the right conditions the response is a
non-monotonic volume recovery, i.e., the volume grows through a
maximum as aging time increases and eventually merges with the V vs. tw
profile obtained upon jumping directly from Tg to Tf.
although it would be symmetric were the response linear.
For the memory experiment, the sample is partially aged at a low temperature Ti
after which T is increased to T=Tf<Tg and
the volume response is measured. Under the right conditions the response is a
non-monotonic volume recovery, i.e., the volume grows through a
maximum as aging time increases and eventually merges with the V vs. tw
profile obtained upon jumping directly from Tg to Tf.
Concentrated colloidal systems exhibit behaviors similar to those of molecular glasses [2]. Aging of colloids has generally been studied by following the evolution of a dynamic response after a shear melting perturbation using techniques such as rheology [3] and diffusive wave spectroscopy [4]. There are no prior works on colloidal systems to investigate the aging behavior relevant to the signatures of structural recovery catalogued by Kovacs [1].
Experimental
Particles of
poly-n-isopropyl acrylamide, PNIPAAM, dispersed in water swell and de-swell
rapidly upon changing temperature [5]. We can change the volume fraction of a
PNIPAAM colloidal system by changing temperature. The volume fraction is 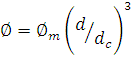 where
where ![]() is the mass fraction and d the diameter. dc
is the diameter of a completely de-swelled (dry) particle. The dynamics of the
PNIPAAM system was probed using multi-speckle diffusive wave spectroscopy [6].
is the mass fraction and d the diameter. dc
is the diameter of a completely de-swelled (dry) particle. The dynamics of the
PNIPAAM system was probed using multi-speckle diffusive wave spectroscopy [6].
Results
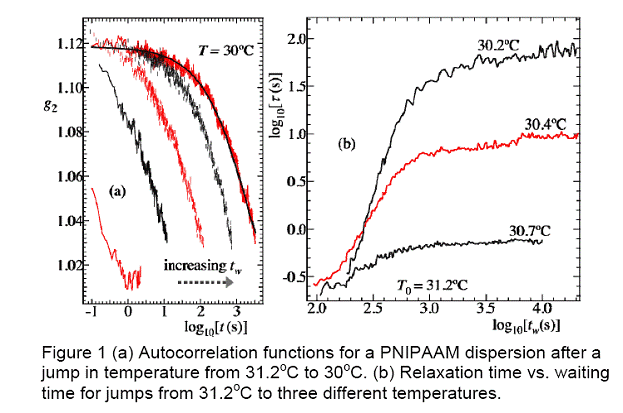
Figure 1a shows the intensity
auto-correlation functions after a down-jump from 31.2oC to 30oC.
We see that ![]() increases with tw. Figure 1b shows
log
increases with tw. Figure 1b shows
log ![]() vs. log tw
for jumps from 31.2oC to different Ts. For each set of data,
there is an initial aging period where t increases with tw
according to a power law:
vs. log tw
for jumps from 31.2oC to different Ts. For each set of data,
there is an initial aging period where t increases with tw
according to a power law: ![]() . At larger tw>1000,
. At larger tw>1000, ![]() levels off.
levels off. ![]() at the largest tw is taken as the
equilibrium value.
at the largest tw is taken as the
equilibrium value.
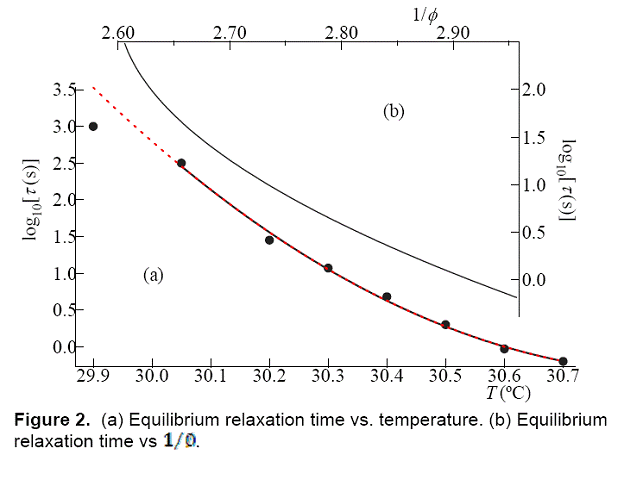
Figure
2a shows that the equilibrium ![]() is greater for lower values of T. The low value
of
is greater for lower values of T. The low value
of ![]() at the lowest temperature is due the sample not
achieving equilibrium. Such behavior is seen in studies of physical aging
behavior in molecular glasses when equilibrium is not attained [7] . With
at the lowest temperature is due the sample not
achieving equilibrium. Such behavior is seen in studies of physical aging
behavior in molecular glasses when equilibrium is not attained [7] . With ![]() we can convert T to
we can convert T to ![]() at each temperature. The equilibrium
at each temperature. The equilibrium ![]() is plotted vs.
is plotted vs. ![]() in Figure 2b.
in Figure 2b. ![]() varies faster than exponentially in
varies faster than exponentially in ![]() .
.
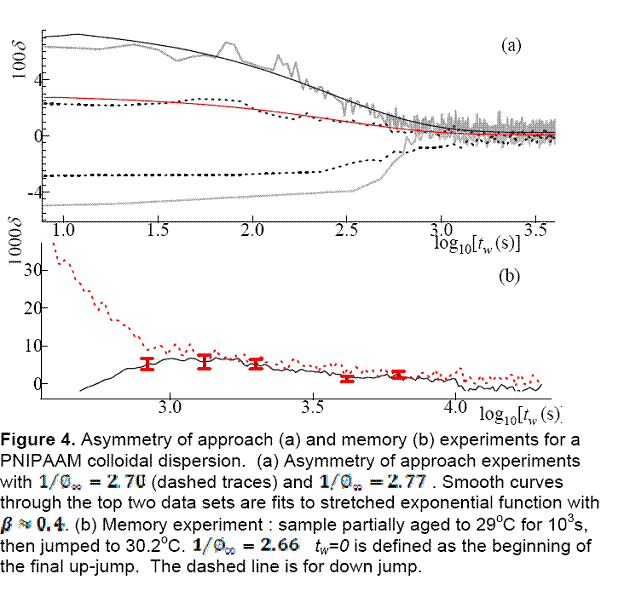
Results for asymmetry-of-approach
are shown in Figure 4a. The upper curves correspond to down-jump experiments
and the lower ones to up-jump experiments. We see that for smaller initial ![]() the asymmetry
is weak and is stronger for the larger
the asymmetry
is weak and is stronger for the larger ![]()
For the memory experiments we partially aged the sample at 29oC for 103s and then jumped to 30.2oC. The response for the up-jump step is plotted in Figure 4b as a solid curve. The dashed curve is the direct jump from 31.2oC to 30.2oC. The magnitude of the memory effect is larger or at least equal to that of Kovacs' data.
Summary
We have shown for the first time, using
thermoresponsive particle colloids, that colloidal glasses exhibit similar aging
signatures to those of molecular glasses. We have demonstrated intrinsic
isotherms, asymmetry of approach and memory effects when the colloidal glass is
subjected to concentration jumps (induced by temperature change histories) that
traverse the colloidal glass concentration.
References
1. A. J. Kovacs, Forschr. Hochpolym.-Forch. 3, 394 (1963).
2. P. N. Pusey and W. van Megen, Nature 320, 340 (1986).
3. G. B. McKenna, T. Narita, and F. Lequeux, J. Rheo. 53, 489 (2009).
4. S. Kaloun et al. Phys. Rev. E. 72, 11401 (2005).
5. M. Balauf and Y. Lu, Polymer 48, 1815 (2007).
6. V. Viasnoff, F. Lequeux, and D. J. Pine, Rev. Sci. Inst. 73, 2336 (2002).
7. A. Lee and G. B.
McKenna, Polymer 29, 1819 (1988).