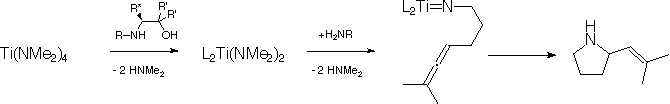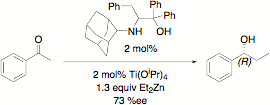Reports: B1
44762-B1 Asymmetric Catalytic Hydroamination of Aminoallenes and Aminoalkenes by Chiral Titanium Amide-Alkoxide Complexes
We have been studying the intramolecular hydroamination of substituted aminoallenes with chiral titanium amino-alcohol derived catalysts. The ligands are prepared in two steps from natural or unnatural amino acids and are enantiomerically pure. We performed the hydroamination reaction catalytically with an enhanced regioselectivity for a-vinylpyrrolidine products over other titanium catalysts, with enantiomeric excesses of up to 16% for our first generation ligands (R' = H). The aims continue to be to improve upon our initial results through systematic ligand tuning.
Asymmetric intramolecular hydroamination of aminoallenes with titanium amino alcohol complexes; R* = CH2Ph or CHMe2; R = iPr, c-C6H11 or 2-adamantyl; 1st generation ligands, R' = H, Me, Bu or Ph.
We prepared ligands with increased steric bulk (R' = Me, Bu or Ph) by alkylating with the appropriate Grignard reagent instead of reduction with LiAlH4 during the ligand synthesis. Except for one derivative that has resisted multiple attempts at synthesis (R = iPr, R* = CHMe2, R' = Ph), we have completed the series and have synthesized and characterized the remaining 17 ligands. Enantioselectivities in the hydroamination reaction were measured for the 17 newly prepared ligands; however, there is not a substantial increase in enantioselectivity with the second generation ligands.
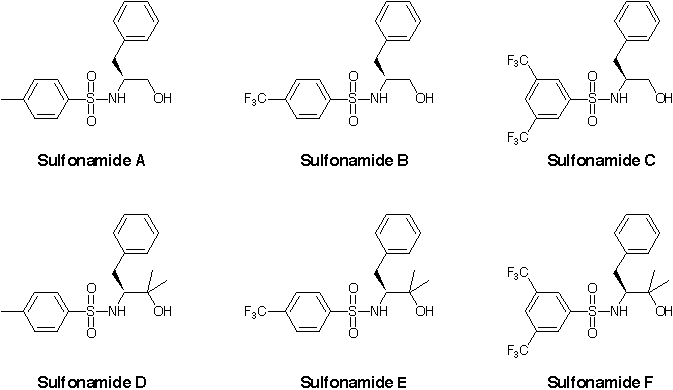
We have prepared six sulfonamide ligands, including four with electron withdrawing CF3 groups and have determined the enantioselectivities of the hydroamination reaction with these new ligands at various temperatures. We were hoping that the increased electron withdrawing character of the sulfonamide ligands would result in an increase in the rate of the reaction. This would allow us to carry out the reaction at a lower temperature and improve enantioselectivity. When carried out at 135 °C according to our standard procedure, we observed 100% conversion, but no significant change in enantioselectivity relative to other amino alcohol ligands. Reducing the temperature resulted in very low conversions.
New sulfonamide ligands for hydroamination
Our ligands and their titanium complexes also catalyze the alkylation of benzaldehyde with diethylzinc. When this reaction is carried out with our first generation ligands (R' = H), we observe enantiomeric excesses below 10% in most cases. However, increasing the steric bulk (R' = Bu or Ph) generally increases the ee values significantly, with values near 75% for our best ligands.
Asymmetric alkylation of benzaldehyde with diethylzinc catalyzed by titanium amino alcohol complexes.