Reports: AC5
46107-AC5 Synthesis, Characterization, and Physical Properties of Periodic Mesoporous Group IV Semiconductors Formed through Zintl Clusters/Surfactant Co-Organization
Nanoporous Group-IV Materials for Applications in Energy Storage
This report discusses two recent results. It first describes electrochemical charge storage in nanoporous silicon made by reduction of tempated porous silica. We also examine templated nanoporous germanium, synthesized by a different route, as a potential material for hydrogen storage. Broadly considered, nanoporous group-IV materials appear to be promising for application in energy storage.
Silicon has been widely investigated in recent years for applications as an anode in lithium ion batteries. Silicon has the highest known storage capacity for lithium (~4200 mAhg‑1). Its lithium capacity is more than 10x larger than that of graphite (372 mAhg-1), which is widely used in commercial lithium-ion batteries. What has prevented the use of Si as an anode material is the enormous volume change, ~400%, associated with the intercalation of lithium to form Li22Si5 and de-intercalation back to Si. This volume change causes cracking and pulverization of the Si, leading to the loss of contact and a continuous decrease in capacity with cycling. Despite a decade or more of research on such topics as nano-sized materials and composite structures, improvements have been limited.
 In
this report, we examine nanoporous silicon generated by magnsiothermal
reduction of periodic, block-copolymer templated nanoporous silica to form
silicon and magnesium oxide. The magnesia is then dissolved in acid to leave
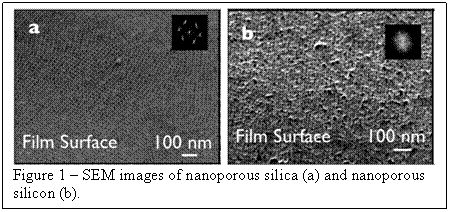
periodic nanoporous silicon. Figure 1 shows SEM images of both the initial
nanoporous silica films and the nanoporous silicon. These films show
homogeneous, 3-dimensional porosity, a fact which is very important for
maintaining mechanical stability of the film upon cycling.
In
this report, we examine nanoporous silicon generated by magnsiothermal
reduction of periodic, block-copolymer templated nanoporous silica to form
silicon and magnesium oxide. The magnesia is then dissolved in acid to leave
periodic nanoporous silicon. Figure 1 shows SEM images of both the initial
nanoporous silica films and the nanoporous silicon. These films show
homogeneous, 3-dimensional porosity, a fact which is very important for
maintaining mechanical stability of the film upon cycling.
When functioning as an anode, the silicon is further reduced according to
Si(s) + Li+ + e- ® LixSiy(s).
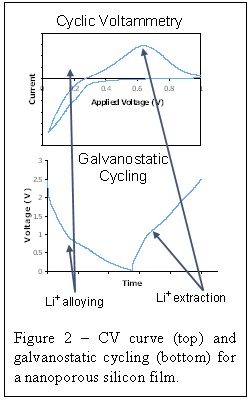 Figure
2 shows a cyclic voltamogram and a galvanostatic charge/discharge cycle for our
porous silicon films. Both alloying of Li and Si and Li+ extraction
can be clearly seen in the plots.
Figure
2 shows a cyclic voltamogram and a galvanostatic charge/discharge cycle for our
porous silicon films. Both alloying of Li and Si and Li+ extraction
can be clearly seen in the plots.
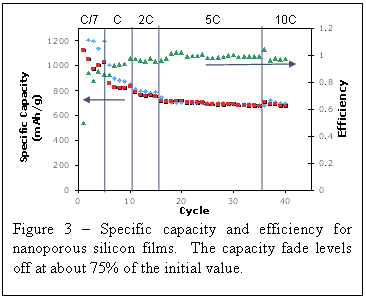
More important than the fact that silicon can be reversibly alloyed with Li+, however, is the stability of the porous silicon over many cycles. Figure 3 shows cycling behavior for one nanoporous silicon thin film. Cycling of these porous films leads to a plateau in charge storage at a value far higher then for bulk silicon films, which often show no leveling off of the capacity as the film crumbles. In addition, the porous nature of these films allow for far greater cycling rates than is possible with dense films. Indeed, the samples show good retention of capacity at rates up to 10C. We postulate that the thin walls in the porous silicon films lessens the time required to diffuse lithium atoms in the alloy, while the periodic nanoscale porosity provides mechanical robustness that prevents failure of the material over many cycles. While we are not achieving theoretical capacity from our silicon films, this work does provide a new avenue for exploration in using nanoporous architectures for achieving that goal.
A second area of research is nanoporous germanium. We have previously shown that Zintl clusters can be used as building blocks for periodic nanoporous germanium. In this case, the porous group-IV material is synthesized directly, rather than by reduction of an oxide. In the synthesis of templated nanoporous amorphous germanium, a Zintl clusters such as Ge94- co-assembles with cationic surfactants to form a periodic composite material with a hexagonal honeycomb structure. The reactive nature of the Ge94- cluster allows for easy conversion to an amorphous semiconductor. Treatment with a mild oxidizing agent like ferrocenium results in destruction of the Ge9 cage structure but retains much of the nanometer-scale periodicity. Oxidation of the Ge beyond ~70% destroys the nanoscale architecture because of the high surface area, but the residual anionic charges can be neutralized with protons, which serve both to complete the oxidation of the Ge and to passivate any remaining dangling bonds. These materials have pore diameters of ~ 2nm and show porosity as high as 500 m2/g as measured by N2 adsorption/desorption.
 Over
the past year, we have made significant improvements in our understanding of
and control over this synthesis. While many improvements have been made, the
key discovery was that weak acids specifically protonated amines could be
used both as the oxidant and as the proton source in the synthesis of these
materials. We can thus replace the old multi-step process where the Zintl
clusters were first oligomerized, the clusters then assembled with the
surfactant, the clusters were then partly oxidized with ferrocenium, and finally
the material was completely oxidized using a proton-exchange resin. In the new
single-step reaction, the Zintl clusters are mixed with the surfactant and a
simple reagent such as ammonium bromide is slowly added to the solution. These
changes have resulted in more periodic material with more open pores, as
measured by N2 adsorption/desportion isotherms. More importantly,
they have allowed us to scale up the synthesis and explore new applications of
these materials, including H2 adsorption.
Over
the past year, we have made significant improvements in our understanding of
and control over this synthesis. While many improvements have been made, the
key discovery was that weak acids specifically protonated amines could be
used both as the oxidant and as the proton source in the synthesis of these
materials. We can thus replace the old multi-step process where the Zintl
clusters were first oligomerized, the clusters then assembled with the
surfactant, the clusters were then partly oxidized with ferrocenium, and finally
the material was completely oxidized using a proton-exchange resin. In the new
single-step reaction, the Zintl clusters are mixed with the surfactant and a
simple reagent such as ammonium bromide is slowly added to the solution. These
changes have resulted in more periodic material with more open pores, as
measured by N2 adsorption/desportion isotherms. More importantly,
they have allowed us to scale up the synthesis and explore new applications of
these materials, including H2 adsorption.
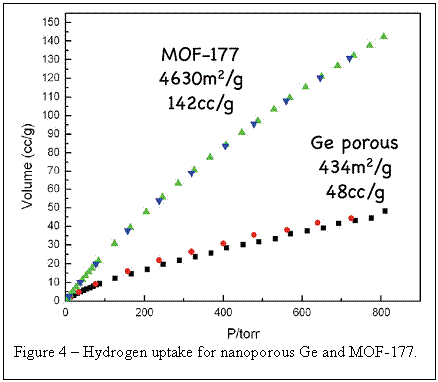
Group IV materials have long been considered for H2 absorption with graphitic carbon receiving the most attention. silicon and germanium, however, have the potential for much stronger interactions with H2 and thus better room temperature adsorption, particularly materials with very small pores like the Ge discussed here. Figure 4 show an example of H2 uptake for our nanoporous Ge at 77 K. A standard H2 adsorption material (MOF-177) is shown for comparison. While the mass normalized data for the porous gemanium is lower than the MOF, the surface area normalized numbers are much higher for germanium compared to the MOF. Recent preliminary variable temperature hydrogen uptake data on our porous Ge suggest that these materials show a large adsorption enthalpy and may be promising new materials for room temperature hydrogen storage.




