Reports: AC7
44247-AC7 Complementary Hydrogen Bonding in Highly Branched Macromolecules: Thermoreversible Supramolecular Architecture for Improved Melt Processibility and Performance
Nature's ability to precisely assemble macromolecules into highly cooperative and functional assemblies provides an inspiration for our proposed efforts. Our research program involves novel synthetic strategy to tailor the relationship between macromolecular structure and non-covalent interactions for the discovery of novel functional, ion-containing macromolecules. Our current research efforts involve the discovery of novel phosphonium cation-containing block copolymers as interesting candidates for ion-conducting membranes and the investigation of nucleobase-containing hydrogen bonding polymers with tunable rheological properties for melt processing and self-healing. The phosphonium cation has received relatively sparse attention as a site for intermolecular interactions, however, the phosphonium cation offers superior thermal stability compared to ammonium cations. The foundation for this program is based on controlled radical polymerization for the preparation of well-defined macromolecules with precisely defined molecular weights, molecular recognition sites, and pendant ionic functionality.
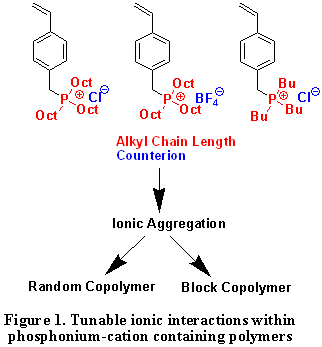
Alkaline fuel cells (AFCs), which are often based on a hydrogen fuel source in combination with a liquid electrolyte such as aqueous potassium hydroxide, offer potentially superior performance compared to other fuel cell systems operating below 200 oC.1,2 Moreover, AFCs provide a more affordable energy solution due to the suitability of less expensive non-noble metal catalysts such as nickel and silver relative to platinum for sulfonated proton exchange membranes.3 Future energy security demands our design of future generations of alkaline anion exchange membranes (AAEMs) with improved resistance to bicarbonate/carbonate formation, enhanced thermal stability for AFC operation at elevated temperatures, increased resistance to hydroxide degradation, and higher membrane conductivities. Although ammonium cation substitution has received considerable attention, investigations of the potential advantages of phosphonium are sparse in the literature.
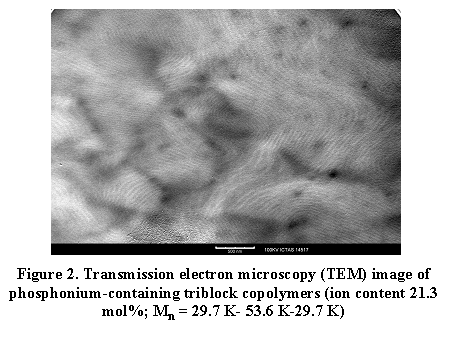
Our research program was catalyzed from the original preparation of a relatively unexplored hydrocarbon-derived monomer containing a pendant trialkylphosphonium cation, which was amenable to controlled free radical polymerization methodologies using our novel difunctional bis-nitroxide initiator. In addition, collaborations with Eastman Chemical and Kraton Polymers revealed the suitability of this novel functional hydrocarbon platform for both adhesive and elastomer technologies, respectively. We have synthesized a series of phosphonium cation-containing block copolymers with external phosphonium sequences and internal acrylic blocks. Figure 2 shows an example of microphase-separated phosphonium-containing triblock copolymers and the formation of lamellar nanostructures are clearly evident. Varying the length of alkyl substituents attached to phosphorus and the size of mobile counteranions enables tuning the strength of electrostatic interactions. Studies have shown that ionic interactions greatly influence corresponding polymer physical properties, such as dynamic mechanical, morphological behavior, 4 and ionic transport mechanisms. Our attention on tailored, self-assembled phosphonium triblock ionomers as model membranes provides structure and ionic transport relationships that promises advances in block copolymer structure for emerging applications.
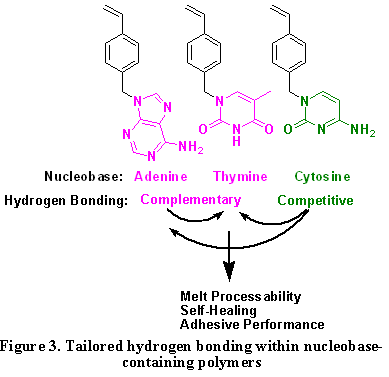
Figure 3 depicts the building blocks for the introduction of pendant nucleobases and the implications of tailored hydrogen bonding on self-healing. The physical cross-links that are derived from microphase separation contribute to self-healing as well as mechanical integrity.5-8 The strength and reversibility of complementary hydrogen bonds are highly dependent on environmental conditions, and dynamic molecular recognition processes for self-healing have not been significantly addressed in the literature.8
Nucleobase-containing linear and branched copolymers exhibited DNA-like melting behavior and a stronger temperature dependence of melt viscosity compared to non-hydrogen-bond containing polymers, suggesting possible advantages in self-healing at relatively low temperatures. The self-assembly of adenine- and thymine-containing polymers serve to mimic the dynamic nature of nucleic acids. The research program has studied complementary nucleobases pairs (adenine and thymine) and also recently introduced cytosine into polymer backbones as hydrogen bonding competitors to achieve tunable hydrogen bonding capacities. Phosphonium cationic guests with the complementary molecular recognition sites enables placement of the ionic sites.
Current efforts are focused on the preparation of all four nucleobases based on styrenic functionality. The four monomers will be used to demonstrate the role of sequenced hydrogen bonding on morphological and rheological performance. Moreover, our efforts will continue to address the new area of "supramolecular click" chemistry wherein the functional group is located on the polymer template using molecular recognition.5-7 An important question arises concerning the selective nature of the functionalization, and the ability for a molecular guest to selectively locate the complementary pair in the presence of other nucleobases is an important aspect of continued research efforts. In addition to phosphonium cationic guests,8 we propose other ionic guests for recognition. The primary principle is the ability to diffuse the associative site from the polymer backbone, and disassociation in the melt step will enable more facile processing of thermoplastic elastomers containing ionic sites.
References:
(1) Coutanceau, C.; Demarconnay, L.; Lamy, C.; L¨¦ger, J. M. "Development of Electrocatalysts for Solid Alkaline Fuel Cell (SAFC)" Journal of Power Sources 2006, 156(1), 14-19.
(2) Varcoe, J. R.; Slade, R. C. T. "Prospects for Alkaline Anion-Exchange Membranes in Low Temperature Fuel Cells" Fuel Cells 2005, 5(2), 187-200.
(3) Varcoe, J. R.; Slade, R. C. T.; Wright, G. L.; Chen, Y. "Steady-State dc and Impedance Investigations of H2/O2 Alkaline Membrane Fuel Cells with Commercial Pt/C, Ag/C, and Au/C Cathodes" J. Phys. Chem. B 2006, 110(42), 21041-21049.
(4) Page, K. A.; Cable, K. M.; Moore, R. B. "Molecular Origins of the Thermal transitions and Dynamic Mechanical Relaxations in Perfluorosulfonate Ionomers" Macromolecules 2005, 38, 6472-6484. ADDIN EN.REFLIST ADDIN EN.REFLIST
(5) Cheng, S.; Mather, B. D.; Long, T. E. "Taking Advantage of Noncovalent Interactions in the Design of Self-Healing Polymers" Polymer Preprints 2008, 49(1), 978-979.
(6) Mather, B. D.; Baker, M. B.; Beyer, F. L.; Green, M. D.; Berg, M. A.G.; Long, T. E. "Multiple Hydrogen Bonding for the Noncovalent Attachment of Ionic Functionality in Triblock Copolymers" Macromolecules 2007, 40, 4396-4398.
(7) Mather, B. D.; Baker, M. B.; Beyer, F. L.; Berg, M. A. G.; Green, M. D.; Long, T. E. "Supramolecular Triblock Copolymers Containing Complementary Nucleobase Molecular Recognition" Macromolecules 2007, 40, 6834-6845.
(8) Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. "Self-healing and thermoreversible rubber from supramolecular assembly." Nature 2008, 451(7181), 977-980.