Reports: B1
46293-B1 Development of Synthetic Tools Using Ynol Ether Ionic Chemistry
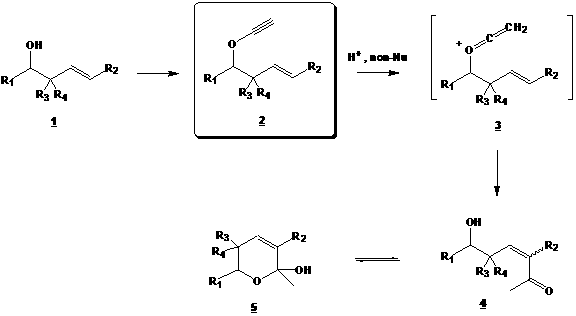
Our objective is to study the intramolecular reaction of homoallylic ynol ethers 2 (see Scheme 1) with electrophiles in non-nucleophilic media. The purpose is to understand the reactivity of ynol ether derived cationic ketene intermediates 3 with strategically placed internal nucleophiles. This strategy should allow us to form unsaturated ketones 4 and/or hemiketal 5.
Scheme 1
This project began with the preparation of alcohols 1 according to Araki's protocol (ref. 1). The latter were transformed to ynol ethers 2 using Greene's method (ref. 2).
In the first year of this project, we observed that one of the R3 and R4 groups must be different from H when preparing ynol ethers 2 from alcohols 1. Indeed, when R3=R4=H, an elimination occured during the process and left us with undesired conjugated dienes.
Consequently, models 1a (R3 = H, R4 = Me) and 1b (R3 = R4 = Me) were prepared and used for the rest of our study.
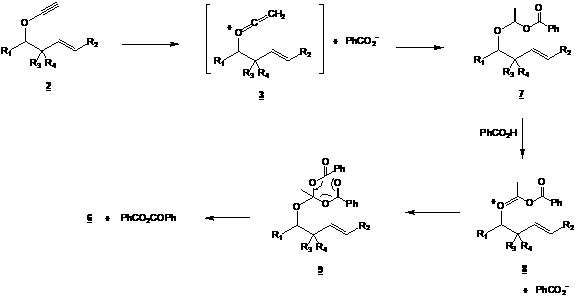
In this second year of the project, we treated models 1a and 1b with Montmorillonite. We thought that this H+ resin would act as non-nucleophilic medium and lead to cationic ketene 3. Results in different solvents, concentrations and temperatures all led to the undesired acetate 6 (see below). We also tried to catalyze the formation of cationic ketene 3 using HgO, Hg(OAc)2 and Ru(p-cymene)Cl2 (ref. 3 and 4). Unfortunately, again, only the acetate 6 was observed in all cases.
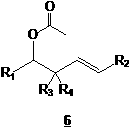
We then study the possibility of transforming the ynol ether moiety to another electrophilic species, a vinyl ester. This vinyl ester could also be a source of cationic ketene, our key intermediate for the formation of unsaturated ketone and/or unsaturated hemiketals. We thus began our study by using the experimental conditions developed by Kita (ref. 3 and 4), known to lead to vinyl esters. One equivalent of ynol ether 2 was treated with one equivalent of benzoic acid in order to produce the desired vinyl ester 7 (see Scheme 2). Again, only the acetate 6 was produced. At first, we thought that production of 6 was due to residual water present in the hygroscopic organic acid. However, we soon realized that the acetate formation was not due to the presence of H2O. Our results rather suggest that the formation of acetate 6 is due to a rearrangement of compound 9 (see Scheme 2), produced by the attack of a second benzoic acid molecule on the electrophilic protonated vinyl ester 8.
Scheme 2
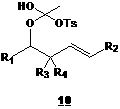
If the mechanism presented in Scheme 2 is indeed taking place, reducing the number of equivalents of acid should reduce the formation of compound 9, thus reducing the quantity of acetate 6 produced thus favoring the formation of vinyl ester 7 (though in yields lower than 100% !). Unfortunately, treating 2 with 0,5 equivalent of benzoic acid led only to the acetate 6. We then treated ynol ethers 2 with more acidic compounds (thus producing a less nucleophilic conjugated base in the reaction medium), Cl3CCO2H and CF3CO2H. Once again, only the acetate 6 was obtained. Using p-toluenesulfonic acid (TsOH) instead of carboxylic acids gave a different result. Indeed, a very small quantity of acetate 6 was produced. However, a new compound was observed with interesting yields (after structure elucidation, we realized that the yields were higher than 50%). A lot of efforts went into characterization in order to find the structure of this new compound. Conclusion came very recently (just before the summer came to an end, time when our workforce (undergraduates) went back to school). Considering all spectral data we gathered for this compound (NMR 1D and 2D, IR, MS, UV), only one structure seemed plausible, hemi ortho ester 10.
We observed that this compound is surprisingly stable for a hemi ortho ester (stable under weak acidic conditions !). Our next move will be to study the formation of this compound. Afterwards, we intend to find out if there are any conditions that would allow the transformation of this hemi ortho ester 10 to a cationic ketene, and hopefully observed alkene attack on this highly electrophilic species.
References
ADDIN EN.REFLIST (1) Araki, S.; Ito, H.; Butsugan, Y. Journal of Organic Chemistry 1988, 53, 1831-1833.
(2) Moyano, A.; Charbonnier, F.; Greene, A. E. Journal of Organic Chemistry 1987, 52, 2919-2922.
(3) Kita, Y.; Maeda, H.; Omori, K.; Okuno, T.; Tamura, Y. Synlett 1993, 273-4.
(4) Kita, Y.; Maeda, H.; Omori, K.; Okuno, T.; Tamura, Y. J. Chem. Soc. Perkin Trans. 1 1993, 2999-3005.