Reports: AC4
46271-AC4 The Oxygen Reactivity of Flavoenzymes: A Structural Approach
The problem
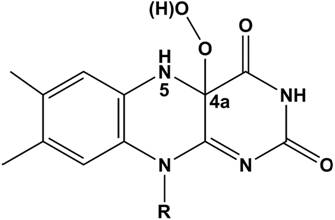
Flavoenzymes catalyze a wide range of reactions essential for maintaining cellular processes. Their striking chemical versatility is largely based on their ability to control the reaction of the flavin cofactor with O2. Three major groups of flavoenzymes can be distinguished. First, the dehydrogenases, that are characterized by poor or no reactivity with oxygen. Second, the monooxygenases, that react very fast with O2, forming a flavin adduct intermediate (C4a-hydroperoxyflavin; Figure 1), which subsequently inserts an oxygen atom into the substrate molecule. Third, the oxidases, that catalyze a rapid two-electron transfer to O2 to produce H2O2, typically with no detectable catalytic intermediates. The reaction of reduced flavins with O2 proceeds through an electron-transfer step that generates a caged radical pair, which is generally thought to be rate limiting. Despite its enormous relevance in biochemistry, the oxygen reactivity in flavoproteins remains a poorly understood reaction. How does the oxygen diffuse? How does the protein modulate the flavin reactivity? What makes the difference among oxidases, monooxygenases, and dehydrogenases? Do proteins preferentially employ single specific pathways or a multitude of routes to optimize the O2 uptake process? As outlined in our original application, we are addressing these problems using various approaches applied to different model systems.
Figure 1. Chemical formula of the C4a-hydroperoxyflavin molecule with the re side facing the reader.
Our approach
We have studied O2 diffusion in two flavoenzymes; the monooxygenase component (C2) of p‑hydroxyphenylacetate hydroxylase from A. baumannii and alditol oxidase (AldO) from S. coelicolor using an integrated computational and experimental procedure. Enhanced-statistics MD simulations (total of 30-40 ns of simulation time) captured the spontaneous (unbiased) diffusion of O2, filling the gap between current sampling times with explicit solvent (~101 ns scale) and measured diffusion times (>102 ns scale). Theoretical predictions were followed by experimental verification.
Oxygen diffusion into C2
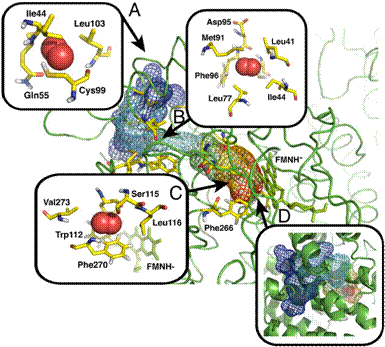
We investigated the diffusion of O2 molecules into the active site of C2, a well characterised monooxygenase. Enhanced-statistics MD simulations were initialized based on the X-ray structure (2.8 Å resolution) of the enzyme bound to reduced FMN (FMNH-) and extended for 30 ns. Out of a total of 500 O2 trajectories, we observe four complete spontaneous diffusion pathways that bring O2 molecules in front of the re‑side of FMNH- (Figs. 1 and 2) starting from random configurations in the bulk solvent. Successful paths typically cover overall distances of about 40-60 Å and display a step-wise behavior from the time of entrance through the C2 protein surface (Fig. 2). The observed spontaneous O2 diffusion matches a model consisting of multiple diffusion paths converging towards Phe270 and Phe266, in front of the re-side of the reduced FMNH- cofactor. Once O2 molecules reach these residues, transient side chain fluctuations allow O2 to further diffuse in order to contact and react with the flavin.
Figure 2. Key steps along an example diffusion path for C2.
Oxygen diffusion into AldO
AldO catalyzes a typical two-electron
flavin-mediated oxidation of a terminal C-OH moiety of
a polyol substrate to the corresponding aldehyde, with the concomitant
reduction of the flavin cofactor. The reduced flavin (FADH-) then reacts
with O2 to form H2O2, which completes
the catalytic cycle. As its crystal structure has been solved at high
resolution (1.1 Å) and the kinetic mechanism has been established by pre-steady
state kinetic analyses, AldO serves as an excellent oxidase prototype. MD simulations
were set-up to capture O2
diffusion before FADH- oxidation.
Out of a total of 500 O2 trajectories, we
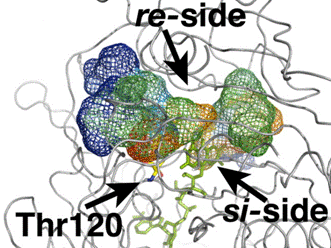
observe five complete spontaneous diffusion pathways that bring O2 molecules
in front of the isoalloxazine ring of FADH- (Fig. 3). Several O2
molecules that initially reside in cavities on the protein surface subsequently
diffuse into the AldO interior. As observed in the C2 monooxygenase
system, these surface cavities typically display at least one hydrophobic
residue, such as
Figure 3. Funnel-shaped
multiple pathways for AldO. For graphical purposes only 3 paths are displayed.
Volume isosurfaces are colored depending on simulation time from blue (entrance
into the protein) to red (end of successful path).
Biochemical insight
In both
simulated systems, distinct and multiple protein-guided O2 diffusion pathways converge toward defined active site entry points,
forming funnel architectures. Our results suggest that multiple diffusion
pathways, converging to a few key residues, are operative and represent a more
effective structural model to combine high specificity and high kinetic
efficiency in oxygen-utilizing flavoenzymes. From a computational standpoint,
we emphasize the novelty of this work in that O2 diffusion
was only solvent- or protein-guided, i.e. no biasing force was employed to
direct the reactants to the protein active sites.
Our MD
simulations indicate that O2 is able
to reach specifically the flavin C4a atom both in the monooxygenase and the
oxidase. The combination of our data with the results obtained by site-directed
mutagenesis indicates that the difference among dehydrogenases, monooxygenases,
and oxidases ultimately resides in the fine modulation of the local environment
embedding the C4a atom; its accessibility, the distribution of charged and
polar groups to favor electron transfer between reduced flavin and O2, and the extent to which the oxygen cavity is capable of stabilizing
the C4a-hydroperoxyflavin.