Reports: G9
47752-G9 Effect of Naphthenic Acids on the Dielectric Response, Particle Aggregation, and Sedimentation Behavior of Asphaltene Suspensions
Introduction
Although several studies exist on the self-association and aggregation behavior of asphaltenes, the interaction of asphaltenes and naphthenic acids, the acidic and basic components of crude samples, are largely overlooked. The overall goals of this PRF program are (1) understanding how the presence of naphthenic acids may contribute to the dielectric response, particle aggregation, and sedimentation behavior of asphaltene suspensions and (2) understanding how the physics, chemistry, and electric potential of the substrate surface affects adhesion of particles, and how deposition may be controlled through surface modification. Year 1 has focused on goal 1. In this effort, the aggregation and sedimentation behavior of asphaltenes in the presence of select naphthenic acids methyl abietate, hydrogenated methyl abietate, 5b-cholanic acid and 5b-cholanic acid-3-one were investigated using dynamic light scattering (DLS), near-infrared spectroscopy (NIR), and molecular modeling.
Experimental
Asphaltene isolation
Asphaltenes were isolated from Shell crude oil samples by a modified n-heptane method. A solution of 1:1 Shell crude oil:toluene was mixed together and sonicated for ten minutes. Next, n-heptane was added to this solution in a 40:1 ratio and sonicated for thirty minutes. This solution was then allowed to stir gently for 24 hours in the dark. The precipitated asphaltenes were then filtered using a 0.2 µm PTFE membrane filter. The collected asphaltenes were then washed three times with ~400 mL, 60o C n-heptane, followed by vacuum oven drying for 24 hours at RT. N-heptane and toluene (HPLC) were used as received. Isolated asphaltene samples were characterized using GPC and LDI spectrometry and found to posses average molecular weights ranging from 500-1000 g/mol. Elemental analysis gave C% 82.52, H% 7.07, N% 1.28, O% 2.00, and S% 1.81.
Asphaltene onset of flocculation determination
A 12.5 mg sample of asphaltenes was suspended in a solution of 45/55 toluene/n-heptane in the presence of 125 mg of naphthenic acid. Solutions were prepared for each naphthenic acid. Methyl abietate, 5b-cholanic acid, and 5b-cholanic acid-3-one were used as received from commercial sources. Hydrogenated methyl abietate was prepared from methyl abietate by a modified hydrogenation procedure from Mori, A. et al.[1] Samples were titrated with n-heptane over a period of 8.3 minutes to introduce 0.5 mL; 24o C sample temperature was maintained by a circulating chiller. After adding n-heptane, the solutions were stirred in the dark for 30 min and then allowed to sit for 5 min before measurement. Particle size measurements were acquired either using a Microtrac Nanotrac Dynamic Light Scattering instrument or a Varian Carey 500 UV-Vis-NIR spectrophotometer, and experiments were run in triplicate.
Calculation of asphaltene/naphthenic acid interaction energies
Asphaltene/naphthenic acid interaction energies were calculated using a molecular mechanics program Discover (Accelrys Materials Studio). Interfacial binding characteristics of asphaltenes and their interactions with naphthenic acids were investigated using molecular mechanics and molecular dynamics simulations using the CVFF forcefield. A representative structure of asphaltenes was obtained from published data [2] for the calculations. An atom based summation method was used with a non-bonded interactions cutoff set to 15.5 Aº, accompanied by a spline width of 5.0 A°and a buffer width of 2.0 A°. The optimized structure for asphaltene was obtained through a series of minimizations originating from multiple starting point geometries. Generally, the interaction energy is estimated from the energy difference, DE, between the total energy of the composite and the sum of the energies of individual molecules as shown in equation 1.
Results and discussion
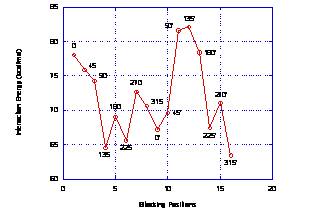
TEM image and DLS results of asphaltene suspensions in toluene indicate average particle sizes of 9 ± 2 nm by DLS and and 10 ± 2 by TEM. The most favorable interaction between asphaltenes occurs at 135o with respect to the other asphaltene molecule, Figure 1.
(a)
(b)
Figure 1(a) Interaction energy versus rotation angle for asphaltene dimer and (b) most favorable interaction energy at 135°
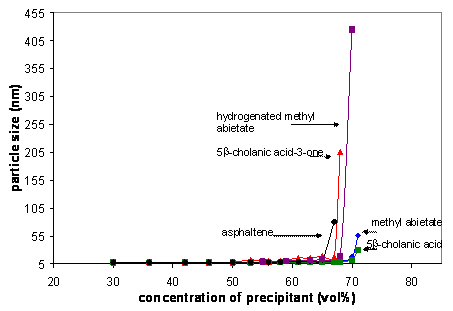
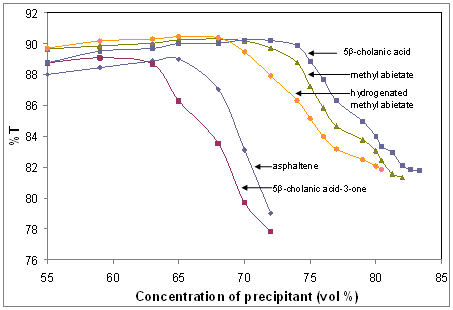
Asphaltenes suspensions
tend to dramatically increase in average particle size, flocculate and
precipitate at ~65 % of added precipitant, Figures 2 and 3. However, in the
presence of select naphthenic acids, flocculation may be delayed to a larger
volume percent of precipitant ~71%. Determining flocculation onsets using DLS are
often criticized due to the fact that asphaltene suspensions typically absorb
at laser interrogation wavelengths; however, recently modified Mie calculations
and a new heterodyne instrument configuration allows for samples containing
absorbing, non-spherical particles at higher concentrations to be investigated.
Figure 2(a) DLS determined precipitation onsets, and
(b) specific titrations with methyl abietate showing reproducibility
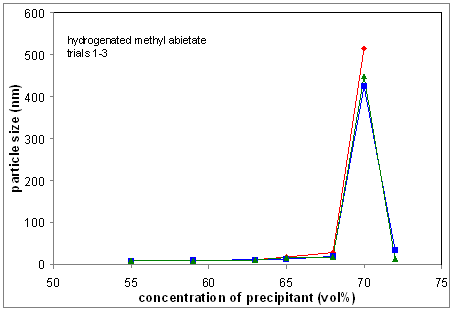
Onsets were also determined using NIR wavelength of 1600
nm, where an increase in light scattering due to the onset of precipitation is
observed. NIR titrations, Figure 3, also show an additive effect. The two
techniques show a very good correlation in detecting the onset of flocculation
for asphaltene and asphaltene/naphthenic acid suspensions.
Figure 3 NIR determined precipitation onsets
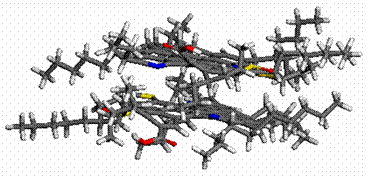
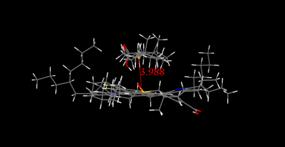
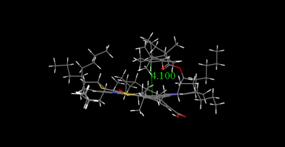
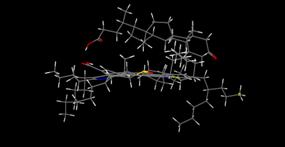
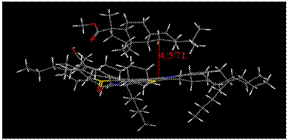
Molecular modeling results supported the interesting trend
revealed in the experimental onset values determined using DLS and NIR
techniques, and minimized energy geometries are provided in Figure 4 (a-d). From
the molecular modeling of asphaltene with methyl abietate and hydrogenated
methyl abietate, minimized interaction distances of 4.10 and 4.57 Å were obtained
and overall interaction energies of 32.47 and -29.63 kcal/mol. This data
shows that the presence of the pi system in methyl abietate offers more
extensive interaction with the asphaltene. In both DLS and NIR, 5β-cholanicacid
presents the largest additive effect, suggesting
a strong association between acidic and basic groups. These finding are
supported by a closer interactive distance of 3.99 Å and interaction
energy of -34.66 kcal/mol. In all
preferred geometries where an additive effect is observed, the asphaltene
adopts a cup-like orientation, with the naphthenic acid residing in the well. However,
minimized geometries obtained for 5β-cholanic
acid-3-one suggests an alternative orientation which is now to large to adapt
the preferred cup orientation leading to a delay in precipitation onset.
Figure 4. Molecular modeling
results describing minimized interaction geometries between asphaltenes and
naphthenic acids