Reports: G5
46510-G5 Application of an Electric Field to Control Wetting of Thin Fluid Films
The ACS-PRF grant (type
G) has helped support two separate projects over the last two years. The focus
of the first project was to understand the importance of particle-obstacle
interactions in a microfluidic separation method called deterministic
hydrodynamics. The goal of the second project was to understand the mechanism
driving electrowetting on dielectric for nanoscale systems. The
ACS-PRF-supported work has led to additional funding from the National Science
Foundation and 3M Corp., as well as long-term collaboration with other
researchers. The Type G grant has also had a large impact on my own career via
support of summer salary. Additionally, the research results have resulted in two
published manuscripts and one more that is submitted.
Project 1) Directional locking
The
potential technological impact of lab-on-a-chip technologies for analytical
chemistry has propelled the need for separation methods that are rapid,
effective, and continuous. One strategy to achieve separation in microfluidic
devices has been the miniaturization of well-established macroscale methods
(such as hydrodynamic chromatography). While effective, these techniques are
characterized (and limited) by the random nature of the pore space, which
implies that the separative displacement of different species is the average
behavior of an inherently stochastic process..
Alternatively, microfabrication has opened the door to new concepts for
separation, such as deterministic hydrodynamics, in which the components being
fractionated become locked into size-dependent periodic trajectories that exhibit
lateral migration. The separation principles involved in deterministic
hydrodynamics are not well-understood: it does not include hydrodynamic or
non-hydrodynamic interactions between the particles and the obstacles, or
possible inertia effects. Thus, it does not include hydrodynamic or
non-hydrodynamic interactions between the particles and the obstacles, or
possible inertia effects. A better understanding of the phenomena was needed to
develop further this promising method.
Achievements.
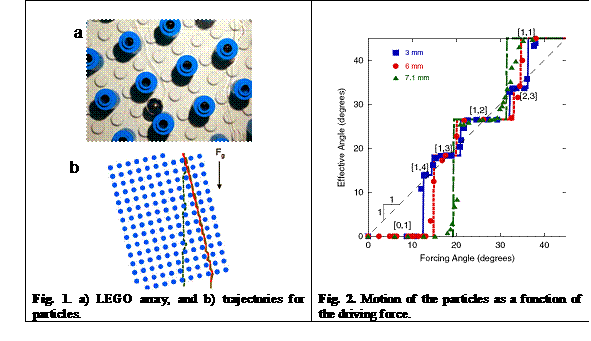
We conducted simple experiments using a large array of obstacles and a uniform
driving force (gravity) to explore the deterministic nature of the
deterministic hydrodynamics separation method. We investigated the motion of
stainless steel balls falling through a periodic array of obstacles created
with cylindrical LEGO® pegs on a LEGO® board and immersed
in a tank filled with glycerol (Fig. 1). We showed that the motion of the
spheres was irreversible and displayed directional locking (Fig. 2). We also
demonstrated that the locking directions could be predicted with a single
parameter that distinguishes between reversible and irreversible
particle-obstacle collisions. These results stressed the need to incorporate
irreversible interactions to predict the movement of a sphere passing through a
periodic array. Interestingly, even though the resulting lateral displacement
is typically small, the periodic nature of the system allows such lateral
displacement to accumulate, thus amplifying the effect into a macroscopic
change in the migration angle. Therefore, by controlling weak, short-range, nonhydrodynamic forces, such as electrostatic forces,
microfluidic devices employing periodic arrays should be able to enhance the
separation of species.
Project 2) Thin
film electrowetting
One of the simplest way to control surface energy is to modify surface
chemistry. In the context of optofluidics and/or microfluidics,
changing the surface functionality is commonly employed as a means to move and
direct fluid on a surface. In porous material, local change in surface
functionality can facilitate or hinder secondary oil recovery. In particle
filtration, local changes in surface charge are suspected to cause unwanted
particle deposition. Our efforts in nanoscale capillarity are to understand,
from a fundamental perspective, the role of an electric field on the stability,
and tunability of liquid bridges in confined
geometry. The surface force apparatus (SFA) was employed as a testbed in concert with external fields. The SFA allowed
the channel height to be varied continuously without changing the surfaces,
allowing systematic and quantitative evaluation of the control of surface
interactions at the nanoscale.
Achievements:
While electrowetting (Fig. 3) is a phenomenon is relatively well-understood for
large (mm) fluid drops, the limits of traditional theories for a fluid drop in
a nanoscale channel have not been tested experimentally. Moreover, the
mechanism at play during electrowetting is not fully understood and the origin
of anomalous features such as contact angle saturation remained debated. Two
different approaches originating from different mechanisms (electrocapillarity
and electromechanics) have been used to describe the
observed change in apparent contact angle in electrowetting. Interestingly,
these completely different theories lead to the same macroscopic response (i.e.
the relationship between contact angle and voltage is the same), which has made
it difficult to unequivocally explain the observed behavior.
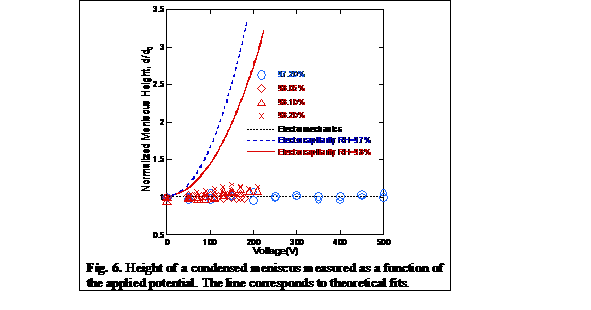
Using the SFA, we have
designed experiments to test the mechanism driving electrowetting (Fig. 4). We
have modified the instrument to allow for external potential control of both
interacting surfaces and used capillary condensation to generate nanoscale
water droplets. Our experiments allowed us to probe contact angle changes
within the first tens of nanometer of a drop, and are not limited by possible
issues caused by contact angle hysteresis. Using this approach we have
unequivocally demonstrated that the real contact angle does not change in
electrowetting experiments. Our results show that there is no measurable change
in the solid-liquid surface energy in EWOD and that the mechanism at play is
electromechanical in nature (see Fig. 5).