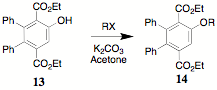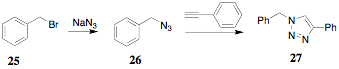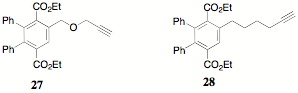Reports: B1
46413-B1 The Synthesis of Novel Indenofluorenes
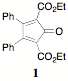
Carboxylated cyclopentadienones (CCPD) react readily in Diels Alder reactions with inverse electron demand (dienophile is electron rich). They present many intriguing possibilities. The reactions outlined in the previous report set the stage for the investigation of 1) additional substitution in the CCPD 2, 5-dicarboethoxy-3,4-diphenylcyclopentadienone 1 ("Orange")by way of substituted benzils, 2) the use of polypropargyl substrates in the preparation of polyterephthalates and 3) the manipulation of polyterephthalic acids as precursors to polyindenofluorenones.
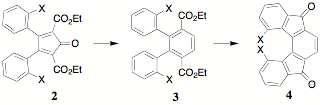
The incorporation of substituents in the benzil portion of CCPDs particularly at the 2 position as shown in 2, is of interest as they are precursors to terephthalates 3 which undergo ring closure to indenofluorenones 4. Steric factors can be used as a probe of the efficiency of the ring closure reaction.
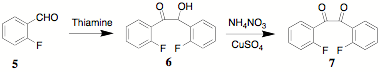
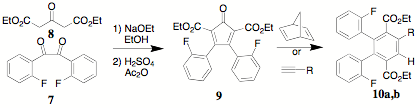
The incorporation of substituents in the benzil portion of CCPDs was investigated by the synthesis of the bis(o-fluoro) derivative 9. A convenient alternative to the use of cyanide ion in the benzoin condensation is the use of thiamine. Thus, 2,2'-difluorobenzoin 6 was synthesized in excellent yield from 2-fluorobenzaldehyde 5. Benzoin 6 was oxidized by the use of ammonium nitrate and copper (CAUTION: nitrogen evolution can be sudden and spontaneous). An alternative oxidation (DMSO, conc HBr) is under consideration. Subsequently, 7 was condensed with diethyl acetonedicarboxylate 8 to yield the fluorinated CCPD 9 in excellent yield. The Diels-Alder reacion of 9 with norbornadiene or a substituted alkyne like 2-octyne yields
terephthalate 10a,b (10a, R = H; 10b, C6H13). The 1H NMR spectrum of 10a indicates the presence of diastereomers. Because of restricted rotation brought on by the fluoro substituents, both the cis and trans isomers can be clearly seen but not conclusively identified. By analogy to similar systems, the trans isomer appears to be present by a 2:1 ratio. Further analysis, both chromatographic and spectroscopic are being pursued.
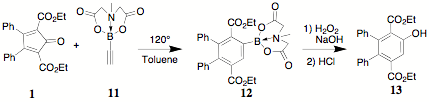
As part of prior work on the cyclodaddition reactions of CCPDs like 1, the incorporation of a phenolic function on the central ring i.e. 13, was desired. Various attempts to incorporate a hydroxyl or ether function on the central ring of terephthalates like 10 (R = OH, OR) were unsuccessful for a variety of reasons – yield unforeseen side reactions, etc.
The announcement by Sigma-Aldrich of the availability of the MIDA ester 11 (Burke, University of Illinois), which exhibits outstanding room temperature stability, was of interest as a reaction partner for 1. Both electronic and steric effects for this combination would be expected to be negative, i.e 1 undergoes reactions with electron-rich dienophiles and the MIDA ester is a large sterically imposing group. The reaction of 1 with 11 yields the highly congested terephthalate 12 in 70% isolated yield. The conversion of 12 to the phenol 13 was accomplished by a standard oxidative pathway.
The determination of optimum conditions for the alkylation of 13 to provide a variety of alkoxy-substituted, phenylated terephthalates 14 is underway.
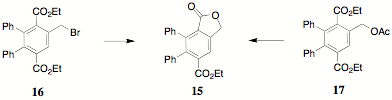
In a previous report, the unexpected appearance of 15 by/during/as a result of either recrystallization of the bromomethylterephthalate 16 or by the equilibration/transesterification of 17 in ethanol was reported. The structure of 15 has been confirmed by X-ray crystallography and will be published soon.
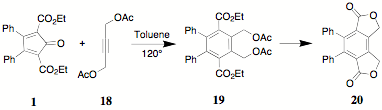
As a companion experiment, the reaction of 1 with 2-butyne-1,4-diol diacetate 18 has been carried out to yield the bisacetate 19. Because of the steric hindrance provided by the two acetoxymethyl groups, the yield of 19 is only about ??% (by 1H NMR). An evaluation of conditions and solvents for the cycloaddition reaction are under study. We anticipate that An equilibration/transesterification similar to the one employed for the conversion of 17 to 15 can be used in the conversion of 19 to 20.
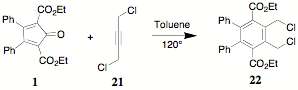
A related reaction between 1 and 1,4-dichloro-2-butyne 21 provides the bischloromethyl derivative 22 in excellent yield.
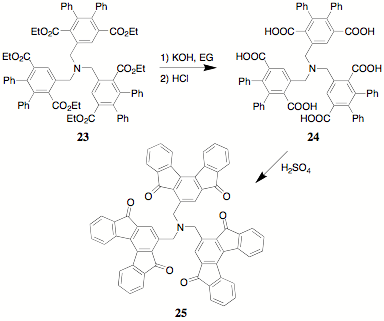
The construction of teathered poly(terephthalates) like 23 is a major objective of this project and prior reports delineated its synthesis. In keeping with our ultimate goal of generating indenofluorenones, the hydrolysis of 23 was carried out in ethylene glycol with potassium hydroxide to yield the hexaacid 24. The characterization of 24 has proven to be a formidable task. The conversion of 24 to the corresponding tris(indenofluorenone) 25 is under study.
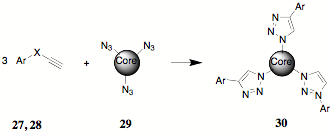
During the synthesis of 23, it was proposed that an alternate approach to the teathered systems might be available through the use of "Click" chemistry and some advantageous chemistry previously reported. The illustration of "Click" chemistry that is evoked most frequently is that of the reaction of a halo compound 26 with sodium azide to form an organic azide 27 which can "Click" with a terminal alkyne 28.
The formation of teathered poly(terephthalates) like 23 or poly(indenofluorenone)s like 25 by click chemistry can be approached by the use of core poly(azides) 29. Alkyne 27 was reported earlier and alkyne 28 is available as well.
A significant change in cycloaddition reaction conditions was provided by the introduction of the Q-tube that is available from Sigma-Aldrich or from the parent company Q Labtech. The Q-Tubeª was created to be an alternative to microwave
synthesis. The Q-Tubeª allows for the use of compounds with low boiling points (highly volatile) and reactions with higher reaction temperatures, higher reaction pressures, and shorter reaction times. The 35mL Q-Tubeª assembly is designed to handle up to 3 g of starting material at 2040C and under 200 psi (the glass tube is rated at 500 psi at 900C). Experiments were carried out to test the capabilities of the Q-Tubeª compared to traditional methods (such as in a RB flask). The Q-Tubeª reactions have shorter reaction times, better yields, and produce cleaner products than conventional methods, particularly open-vessel methods. The use of the Q-Tubeª has become the de-facto standard cycloaddition method.
The reactions outlined in this report add to the knowledgebase of reactivity and reaction diversity for CCPDs, namely 2, 5-dicarboethoxy-3,4-diphenylcyclopentadienone 1 ("Orange"). The construction of arrays of terephthalates as in 23 or indenofluorenones as in 25 and 30 is well underway.