Reports: G3
47820-G3 Iridium (III) Complexes for Aerobic Oxidation
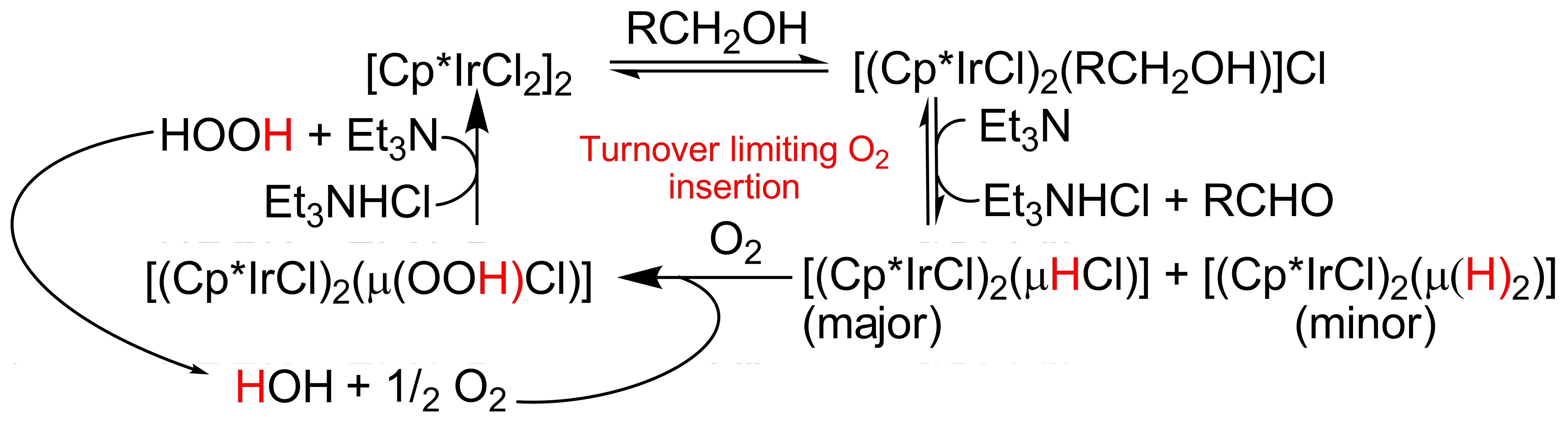
In the current grant period we have made two major discoveries with respect to catalytic oxidations. A novel catalytic system for the aerobic oxidation of primary and secondary alcohols was presented. For these reactions we employed the commercially available catalyst [(Cp*IrCl2)2] with O2 as the terminal oxidant in the presence of catalytic amounts of Et3N. A new mechanism for the Ir catalyzed aerobic oxidation was presented that suggests that the transition metal maintains its (+3) oxidation state throughout the entire catalytic cycle (Figure 1). Evidence in support of this mechanism includes (1) the demonstration that O2 is needed for catalytic turnover, (2) kinetic data from oxygen uptake experiments consistent with the proposed mechanism, (3) kinetic isotope and isotopic labeling data that implicate Ir hydrides as key intermediates in the catalytic reaction, (4) the identification of an Ir hydride, as a key and kinetically competent intermediate in the catalytic cycle, and (5) the identification of the reaction of this iridium with O2 as the turn over limiting step in the reaction. These results have aided in the development of more efficient systems for aerobic oxidations with Ir and other transition metals.
Figure 1. Mechanism for aerobic oxidation with Ir(III)
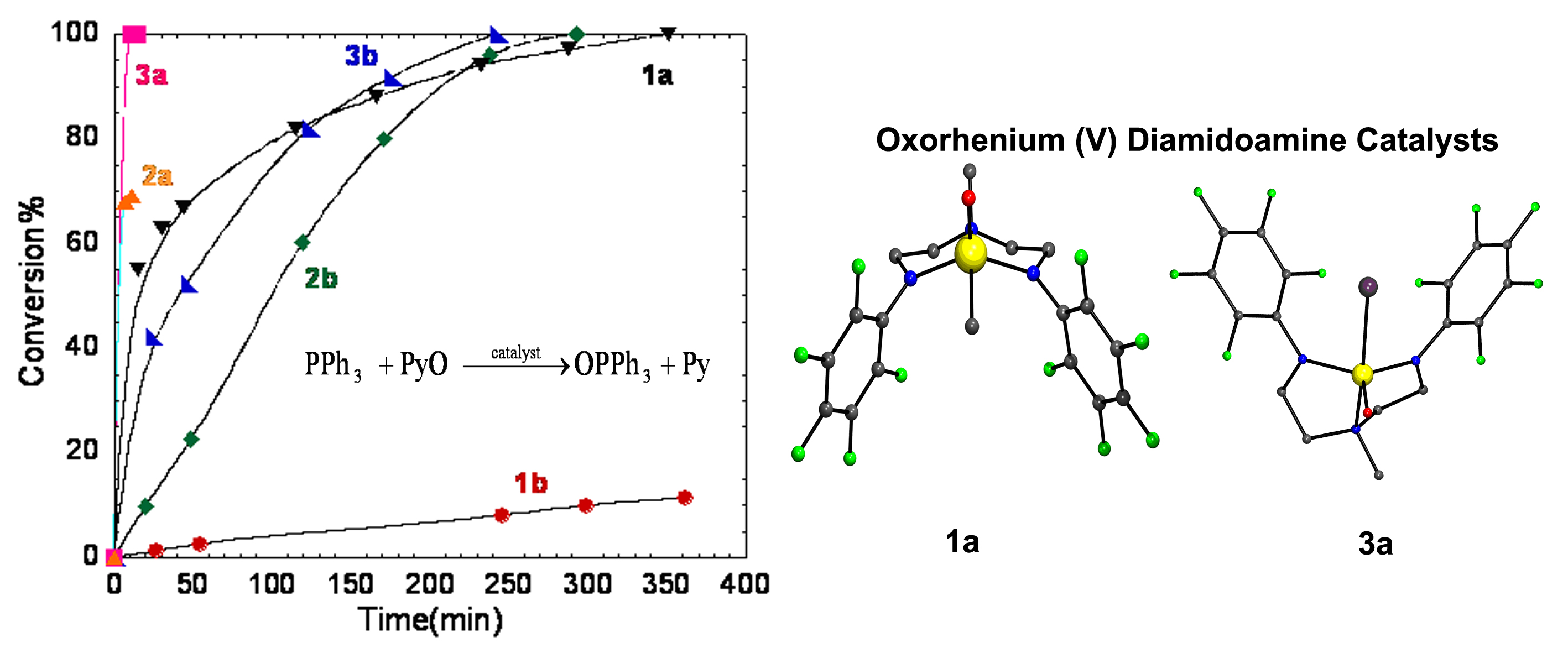
We have also demonstrated that oxorhenium complexes of the form [Re(O)(X)(RNCH2CH2)2N(Me)] (X = Me, Cl, I, R = mesityl, C6F5), incorporating diamidoamine ancillary ligands, can be effectively tuned for oxygen atom transfer reactions by varying the electronics of the amido substituents and the nature of the X ligand attached directly to the metal. Catalytic OAT is favored by electron-withdrawing substituents on the amido nitrogens and poor s donors attached directly to the metal. The rate of oxygen atom transfer in these complexes is governed by isomerization of the metal complex rather than oxidation of the metal. The data presented suggests that the catalytic OAT reactions presented proceed via the generation of an electrophilic high-valent Re oxo species. The catalytic efficiency of these complexes is thwarted however, by the high reactivity of this species and its susceptibility to hydrolytic degradation.
Figure 2. OAT catalysis with Re (V)
During the grant period three undergraduate students, one graduate student and one post-doctoral associate was supported by the grant.