Reports: B10
44743-B10 Glow-Discharge Plasma as a Synthetic Medium for Nanocrystalline Non-Molecular Metal Oxides
Introduction
The plasma synthesis project directly benefitted from ACS-PRF funding from 2006-2008, which allowed the progress detailed in previous narrative reports. Although funding for faculty and students was allocated over the summers of 2006, 2007, and 2008, we were able to continue the work on this project over 2009 on a more limited basis. The focus this year was in redesigning the cathode to increase the rate of product formation and deposition. Several different cathode designs were tried and products were characterized for stoichiometry using atomic absorption spectroscopy and phase composition using powder x-ray diffraction.
The Plasma Reactor and Associated Systems
We focused on modifying the shape and reactant delivery scheme of the cathode this year. The hollow cathode design used in previous years did not address the major problems associated with the plasma synthesis: specifically, low product formation rates and/or product deposition rates. Although, as reported previously, hollow cathode design improvements did result in a more spatially directed plasma, a more concentrated deposition of product on the substrate, and improved product deposition rates, we remain limited by poor reproducibility and small product yields. We continue to successfully synthesize metal and mixed metal oxides, but product yields, stoichiometry, and morphology vary significantly from sample to sampledespite the important progress previously reported. We are currently working creative cathode designs to address these difficulties.
The overall plasma system, including the important enhancements made over the grant period continues to function very well. Specifically, the electronic mass flow control of the plasma gases, argon and oxygen, have vastly improved the control and stability of the plasma gas composition and flow. The MKS type M100B mass-flow controllers and M10MB mass-flow meters allow excellent stability and reproducibility in gas pressure, flow, and mixing. We remain confident that the problems in product formation (described above) are not derived from control over the plasma gases. Moreover, we continue to reap productivity benefits from the second plasma chamber that reproducibly behaves like the first plasma system with respect to gas control and voltage-current relationships. Therefore our continuing work will focus on redesigning the plasma cathode and reactant delivery system to improve product yield and control over product stoichiometry, and product morphology.
Formation and Characterization of Products
As previously reported, we have made approximately 40 different samples of metal oxides from the plasma system over the grant period. These products have been analyzed for stoichiometry via atomic absorption spectroscopy, phase composition via powder x-ray diffraction, and in several cases, morphology via optical and atomic force microscopy. However, in 2008-2009, we have focused on exploring different methods of introducing reactants into the plasma and different cathode configurations. For example, we explored a cup cathode configuration (see Figure 1) and allowed the system to run for 24-48 hours. Products accumulation was then determined by mass measurements. The goal was to find a configuration that would allow reproducible formation of more than 100 mg of product in about 24 hours (or less). Unfortunately, we have not been able to realize that goal, but we are continuing the endeavor. We continue to form copper oxide materials, but reliable characterization is problematic with the low yields realized to date. We are hopeful that continued work on the cathode design will lead to higher and more reproducible yields and more consistent and predictable product stoichiometry.
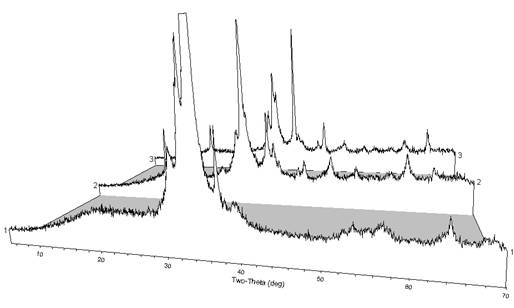
We have analyzed many of the plasma products with powder x-ray diffraction, which typically reveal a crystalline phase of CuO (tenorite) with varying additional peaks and an amorphous phase, as has been typical in previous years (see Figure 2). The variation in peak profile probably represents differences in product composition, purity, particle size, and crystallinity. Ongoing work is focusing on cathode design as a parameter to control product composition, morphology, and yield.
Figure 1. The original cup design for the cathode. Reactants are loaded inside the glass cup on top of the conductive cathode.
Figure 2. Powder XRD patterns from three product films formed from plasma synthesis using different cathode designs. Some peak 2θ positions are consistent with CuO, but the patterns also include unidentified peaks and some amorphous character.