Reports: AC6
47059-AC6 Probing the Metal Activation of C-H Bonds Using High-resolution Mid-infrared Laser Spectroscopy
I. Motivation
The underlying query of metal activated chemistry, whether being in the solid-, liquid-, gas-phase, is role of the electronic state of the metal atom or metal center in controlling chemistry. A coordinated theoretical and experimental investigation of simple gas-phase chemical reactions involving transition metal atoms is an effective route for elucidating this molecular level mechanism for the unusual catalytic activity of transition metals. Here the experimental detection and characterization of proposed gas-phase products formed by the oxidative addition reaction (Eq.1) of naked transition metal atoms with methanol has been undertaken:
M+CH3-OH®CH3-M-OH®CH3M, MOH, CH2M, H2 + (1).
The ephemeral nature (typical concentrations of < 104/cm3) of the products requires an exceptionally sensitive spectroscopic technique. We have developed a sensitive, high resolution, infrared absorption spectrometer for this investigation. Infrared spectroscopy has numerous advantages over electronic and microwave spectroscopy, but traditionally lacked sensitivity. To date there has been no reported high-resolution infrared spectroscopic studies of transient metal containing molecules.
II. Accomplishments
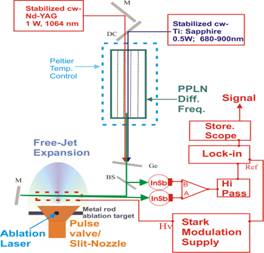
A highly sensitive, laser-based, high-resolution Stark-modulated spectrometer (Figure 1) operating in the 2.8-3.4 mm spectral region has been developed following the Nesbitt design [1]. Unlike other groups, we have implemented a Stark modulation/phase sensitive detection scheme similar to that traditionally used in microwave spectroscopy.
Fig. 1 A block diagram of the high-resolution, mid-IR spectrometer under development. A cold sample (Trot< 10K) of molecules will be generated in slit nozzle supersonic expansion. The monochromatic IR radiation is generated periodically poled lithium niobate (PPLN) crystal. Dual balanced beam detection, high pass filtering, and Stark modulation/phase sensitive detection are employed to enhance sensitivity and spectral simplification.
This approach enhances the sensitivity and also
provides molecular selectivity because it preferentially detects
molecules that have non-zero electric dipole moments. As a test we recorded portions of the OH
stretching fundamental band, n1, of CH3OH in the A: J0,J
®J1,J-1 Q-branch region (Figure 2).
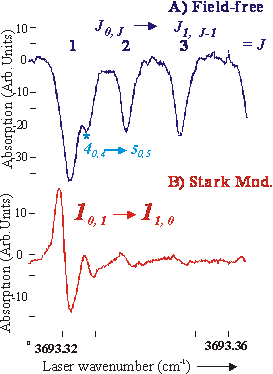
Fig. 2 High-resolution infrared absorption in the OH stretching fundamental band, n1, of a CH3OH sample seeded in a supersonic expansion of argon. A) Field-free spectrum (upper half); B) Stark modulation spectrum. The field-free signals is an 80 average of the pulse expansion where as the Stark modulation is for only 20. Estimated total path length of 3 cm. The line width of approximately 80 MHz (FWHM) is due to residual Doppler broadening. An estimated rotational temperature of approximately 40 K is obtained from the relative intensity of the field-free spectral features.
Our initial target metal containing molecule
for detection has been CuOH generated from the reaction of ablated copper with
a CH3OH/argon mixture. CuOH is amongst the most thoroughly studied
transition metal containing molecules. The electronic [2,3] and pure rotational
[4] spectra have been reported. In Ref. 4 the centrifugal distortion constants
were used to determine the harmonic force field from which the OH stretching
fundamental band, n1,
has been estimated to have a harmonic frequency
w1
of 3738 cm-1. A recent high
level ab initio calculation [5]
predicted w1
of 3856 cm-1 and a dipole moment of
3.98 D. We performed extensive searches in the 3720 cm-1
to 3860 cm-1 spectral region but were unable to detect absorptions. Possible reasons include: a) the laser
ablation scheme produces too low of concentration; b) the transition are outside
predicted spectral range; c) Stark modulation is ineffective because the dipole
moment, m,
is too small.
In order to identify reasons for lack of
detection, an optical spectroscopic investigation of CuOH using the very
sensitive laser induced fluorescence (LIF) detection scheme is being pursued.
The initial results are given in Figure 3.
The ablation/reaction scheme does produce sufficient concentration for
LIF detection.
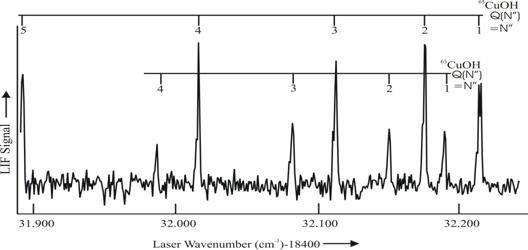
Figure 3 A portion of the field-free LIF spectrum of the We are currently performing optical Stark spectroscopy to
determine the dipole moment to ascertain whether Stark modulation is viable. We
will also record and analyze the high resolution dispersed fluorescence
spectrum to get a more accurate estimate for w1. To evaluate
the relative sensitivity of absorption technique compared to LIF detection we
will re-record the spectrum of Figure 3 using transient frequency modulation absorption
spectroscopy [6]
III. Summary
High-resolution
infrared spectroscopic studies of transient metal containing molecules have not
yet been accomplished by us or others. A series of systematic diagnostics using
CuOH are being performed to identify, and hopefully correct, the limitation. It
has become evident that the narrow tuning of the spectrometer is a serious
limitation for this class of molecules because of the large uncertainty in the
transition frequencies. A coordinated program involving very high-level
predictions and the use of other experimental data is essential.
References
1. C.
M. Lovejoy, and D. J. Nesbitt, High Sensitivity, J. Chem. Phys. 1987 86,
3151-3165.
2.
C. N.
Jarman, W. T. M. L. Fernando and P. F. Bernath,
J. Mol. Spectrosc. 144 286-303
(1990).
3.
C. Tao,
C. Mukarakate and 4.
C.J. Whitham,
H. Ozeki, and 5.
S. Wang, A. Paul, N.J. DeYonker, Y. Yamaguchi, and
H.F. Schaefer,III. J. Chem. Phys. 123, 014313/1-014313/13 (2005).
6. T.C. Steimle, M. L. Costen, G.E. Hall, and T.
J. Sears, Chem. Phys. Lett, 319, 363-367
(2000).
![]() ¬
¬
![]() (000-000) transition of CuOH. A molecular beam sample was generated by skimming the products of an ablated copper metal + CH3OH/Ar free jet expansion. The individual spectral features have a linewidth of <50 MHz . Stark studies of the Q(1) of the 65CuOH isotopologue are in progress.
(000-000) transition of CuOH. A molecular beam sample was generated by skimming the products of an ablated copper metal + CH3OH/Ar free jet expansion. The individual spectral features have a linewidth of <50 MHz . Stark studies of the Q(1) of the 65CuOH isotopologue are in progress.