Reports: DNI7
49218-DNI7 Assembly of Organometallics in Polymeric Templates for Metal Coated Mesoporous Materials
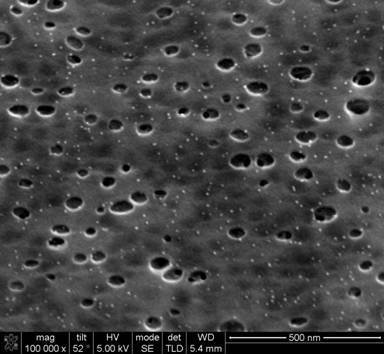
The assembly of surfactants, carbonizable precursors and organometallic compounds were examined using SEM and TEM. Several different carbonizable precursor routes were explored: (1) in-situ reactive where phenol/resorcinol/phloroglucinol and formaldehydye are polymerized during self-assembly, (2) post polymerization where phenol/resorcinol/phloroglucinol and an acid are initially added to the precursor solution and the aromatic alcohols are polymerized using formaldehyde vapor, and (3) non-reactive where an oligomer of phenol-formaldehyde (PF) is added as the carbonizable precursor. Route (1) results in phase separation of the templating solution and a very limited shelf life for the solution prior to solidification. For this later reason, the other two routes have been explored in more detail. The second route is limited to thin films as diffusion of the formaldehyde is required for polymerization. One issue with this approach is that the aromatic alcohol can crystallize partially during annealing and polymerization with formaldehyde; this crystallization appears to lead to phase separation and formation of large pores. An example of this method is shown below in Figure 1.
Figure 1. SEM image of porous carbon-vanadium oxide film formed using route (2). Bright dots are vanadium oxide nanoparticles formed in-situ and dark regions are the pores
The pores in this case are large (25-75 nm) and polydisperse. The organometallic compound, vanadium acac in this case, is decomposed during carbonization of the resorcinol-formaldehyde (RF) resin and removal of the surfactant. This yields 5-10 nm nanoparticles of vanadium oxide (oxygen is presumably provided from the RF resin). Due to the disordered nature of this porous nanocomposite, it is difficult to ascertain the location of the nanoparticles in reference to the pores. Thus another method based upon the co-assembly of PF oligomer, surfactant and organometallic has been explored in attempts to form ordered mesoporous nanocomposites. Figure 2 illustrates the well ordered morphology that can be obtained using this route. In this case, cobalt acac is used as the organometallic precursor. RBS analysis is utilized to confirm the presence of Co as CoO2 within the matrix of the material. It is interesting to note that large nanoparticles of CoO2 are not readily visible in the TEM micrograph.
Figure 2. TEM image of porous carbon-cobalt oxide film formed using route (3). In this case, a highly ordered mesostructure is formed.
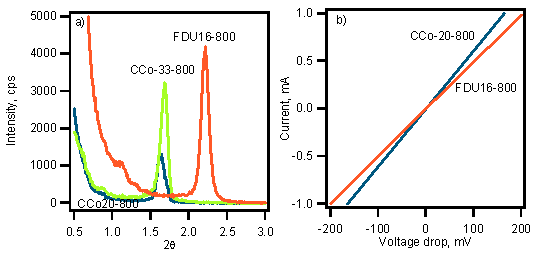
Figure 3 provides additional evidence for the incorporation of CoO2 throughout the material as the XRD diffraction peak location is not shifted as significantly when CoO2 is included in the material in comparison to the neat carbon analogue. The nanostructure during carbonization does not contract as significantly when Co is present; the increased mechanical strength in comparison to the PF resin at high temperature is likely responsible for this characteristic. Additionally as shown in Figure 3b, the conductivity of the mesoporous nanocomposite containing the conductive CoO2 shows a 30 % increase in conductivity in comparison to the pure carbon analogue. These results suggest the ability to disperse metal oxides within ordered mesoporous carbon frameworks with this approach. Future work will focus on examining the morphology in more detail using high resolution STEM and local EDS measurements. Additionally, the impact of the metal center and ligands on the morphology will be systematically investigated.
Figure 3. (a) XRD comparison of mesoporous carbon (FDU16-800) and analogous nanocomposites containing 20 and 33 % Co. A significantly larger d-spacing is maintained for the nanocomposites. (b) Impact of CoO2 incorporation on electronic properties as measured by I-V characteristics.