Reports: AC5
44510-AC5 Surface Reactivity of Radicals and Ions During Plasma-Catalytic Removal of Nitrogen Oxide (NOx) Pollutants
Fig. 1: CCD image of NO LIF signal (intensity rises from blue to white).
The primary focus of this project is to elucidate fundamental chemical processes occurring at catalyst surfaces during plasma-catalytic removal of nitrogen oxides (NOx), beginning with nitric oxide (NO). Mechanisms for NO removal were investigated using the imaging of radicals interacting with surfaces (IRIS) technique, which examines the steady-state reactivity of plasma-generated species at catalytic surfaces. In the final funding cycle, we continued our fundamental gas phase density studies using optical emission spectroscopy (OES) and laser-induced fluorescence (LIF, Figure 1) and extended our studies to include vibrational temperature studies of NO in various gas mixtures. We have expanded to other areas of plasma pollution control, namely detection of organic contaminants in water streams.
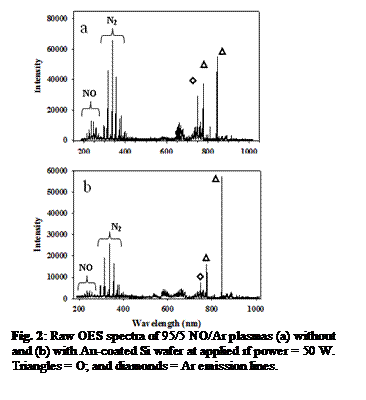
Fig. 2: Raw OES spectra of 95/5 NO/Ar plasmas (a) without and (b) with Au-coated Si wafer at applied rf power = 50 W. Triangles = O; and diamonds = Ar emission lines.
OES Studies.
Experiments in this funding year focused on using platinum and gold with
silicon wafers as controls. Notably,
Au-coated Si wafers were most efficient in removing NO from plasma mixtures (e.g.
NO, NO/Ar, or N2/O2/Ar). As seen from Fig.
2 OES spectra, the presence of Au significantly decreases the NO signal at the
lowest applied rf power (P) of 50 W. At P
> 50 W, NO is effectively eliminated, most likely because higher P effectively dissociates molecules in
the system, and even though recombination reactions can reform NO, dissociation
can lead to formation of alternate gas-phase species. Clearly control of the process gases and
their relative concentrations, along with the overall power and catalyst type
is required to achieve viable plasma processes for removal of NO.
Introduction of water vapor or
methane introduces complexity and a wider range of plasma species that can
affect [NO] through gas-phase reactions.
Although N and O can recombine to form NO, the rate constant for this
reaction is lower than that for forming NO and H via reaction of N atoms with
OH. Additional reactions involving
nitrogen atoms have been proposed as important to either NO destruction, or formation
of NO under conditions high oxygen concentration. Addition of water promotes formation of NO,
perhaps via the loss of singlet oxygen atoms through reaction with H2O
to form OH radicals. Without H2O
(or a hydrocarbon) in the system, NO can be removed via reactions with vibrationally excited singlet N2. Similar trends were observed with methane
addition.
Rotational and Vibrational Temperatures.
Knowledge of energy partitioning between different species is important to an
overall understanding of the chemistry occurring in our plasmas. Thus, this year we have expanded our rotational
temperature (ΘR) database, Table 1, and added vibrational temperature (ΘV) measurement of
NO in our gas mixtures, Table 1. These
data were collected utilizing LIF and OES spectroscopy. For all systems, ΘR does not
change appreciably with P or with gas
mixture.
Table 1. Rotational Temperatures for NO in Different Plasmas (K)
Applied rf power (W)
NO (100%)
NO/Ar (12:88)
NO/Ar (50/50)
NO/H2O (80/20)
NO/CH4 (80/20)
N2/O2/NO (70/20/10)
N2/O2 (90/10)
25
356 (12)
318 (10)
322 (13)
350 (37)
317 (36)
50
342 (12)
313 (9)
327 (24)
318 (20)
328 (24)
323 (40)
75
366 (15)
321 (32)
325 (15)
312 (10)
322 (15)
100
366 (20)
313 (13)
332 (10)
332 (10)
328 (20)
325 (18)
125
368 (34)
339 (25)
320 (18)
317 (15)
320 (13)
150
366 (30)
348 (24)
332 (10)
328 (20)
328 (10)
323 (28)
320 (12)
200
348 (13)
317 (8)
NO vibrational
temperatures, ΘV, were determined from OES spectra, and ranged
from 1400-1700 K. ΘV is
not dependent upon gas mixture or substrate, but values increasing slightly with
P.
OES spectra were also used to determine ΘV(N2)
and again, there was little dependence on plasma or substrate type, but ΘV(N2)
values are much cooler, ~400-500 K, suggesting NO is rotationally thermalized, but that vibrationally
hot NO persists. The lower values for ΘV(N2) suggest vibrationally hot N2 reacts or is quenched, most
likely via reaction to form NO. Notably,
ΘV(N2) is lower than
predicted, suggesting vibrational-translational
energy transfer occurs more rapidly than
NO formation reactions in the systems studied. Further work
currently underway focuses on the use of ceramic oxides as catalytic surfaces.
Organic Contaminant Detection and Removal. We expanded our studies to include plasma
pollution control, including detection and removal of organic contaminants from
water sources. This work has continued
in the final year of the project. Although large volumes of water may not be
treatable with plasma methods, the ability to detect contaminants in ultrapure
water sources (e.g those used in the microelectronics
industry) was also a goal. Among the
many potential contaminants of water sources, those associated with fuel
oxygenate additives such as methyl tert-butyl ether (MTBE) are of significant concern as MTBE
readily partitions into aqueous phases.
We used our inductively-coupled rf
plasmas for detection of methanol and MTBE in water samples using OES as the
detection method. Using emission from
CO*, a detection limit of 0.01 ppm was determined for
each organic contaminant. Complementary
mass spectrometry data were also collected to explore decomposition mechanisms
for both CH3OH and MTBE.
Specifically, we found that CH3OH decomposition is achieved
primarily via an oxidative dehydrogenation mechanism, whereas MTBE abatement
occurs via both decomposition and oxidation mechanisms. This work has resulted in one manuscript that
has been submitted for publication and was provisionally accepted with minor
revisions.
Summary/Impact.
We have further explored plasma-catalytic processes involving NOx removal, including additional gas-phase
characterization of NO and expanded the scope to include other projects with an
environmental focus. Ms. Michelle Morgan, completed her M.S. degree in 2009 (Thesis title: Gas-phase
and Surface Analysis for Exploration of Plasma Catalytic Reduction of NOx).
Ms. Kristina Trevino, was responsible for the water
remediation project. This work was
presented at National and Regional AVS meetings; two manuscripts (one published,
one under review) have resulted with two more in preparation.