Reports: AC5
46837-AC5 Alkane C-H Bond Activation in Hydrocarbon Catalysis with Solid Acids
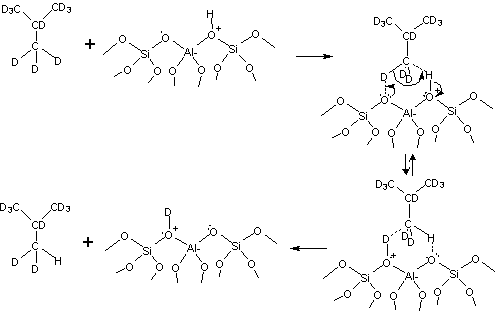
The systematic conversion of hydrocarbons to desirable fuels and chemical feedstocks through heterogeneous catalysis is a continuing goal for the modern petrochemical industry. Increasingly, economic and environmental demands require flexibility in the choice of reagents, catalysts, and controllable reaction selectivities. While reaction and process engineering is the traditional route to provide such flexibility, a fundamental molecular understanding of specific reaction events involving primary and secondary reaction species, as well as the molecular role of the catalyst itself, is the only way to generate new, robust chemistries that provide desirable conversion, selectivity, and lifetime. In our PRF-funded work, we have shown by in-situ experimental NMR techniques that non-classical or weak C-H to O hydrogen bonds are the critical first step in alkane C-H bond activation over solid acid catalysts. In addition, a major conclusion regarding the relationship between catalyst structure and alkane reactivity involves the ease with which the catalyst structure can solvate the hydrogen-bonded transition state, of the type shown below.
Currently, a major need involves catalysts which have solid Bronsted acidity, like the scheme outlined above, but which can transform molecules much larger than the small organic molecules traditionally reformulated by microporous zeolite catalysts. Catalysts of this type, often called mesoporous materials, have larger channel/pore diameters, and are well known in their purely siliceous compositions. However, the degree to which mesoporous materials can present stable solid acidity to large, bulky organic reagents is unclear as there are many schemes proposed in the literature for their creation and use. During the most recent ACS funded period, we have extended our earlier findings to this important area, with primary focus on the incorporation and molecular level characterization of acidic mesoporous materials. Using methods described in the literature, we prepared MCM-41 and then subsequently carried out grafting reactions to incorporate aluminum onto/into the channel walls and compared this material to an SBA-15 preparation where aluminum was directly incorporated into the primary synthesis step, resulting in an Al-SBA-15. The key question, which has not yet been resolved in the literature, is the relative incorporation of the aluminum into stable, active bridging hydroxyl groups of the type known to constitute solid Bronsted acidity. This approach was particularly beneficial to the students, as it allowed them to learn catalyst synthesis and preparation methods, and also exposed them to state of the art theory and practice for modern characterization methods.
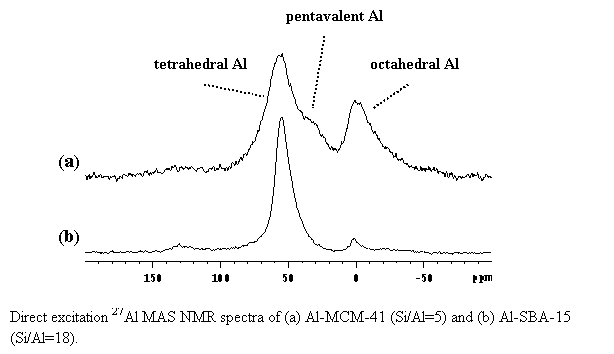
The following figure is one result of many that demonstrates that aluminum incorporation to create solid Bronsted acid sites is efficient and stable for the case of a direct synthesis Al-SBA-15 catalyst, while post-synthetic methods for transforming siliceous MCM-41 into an acidic Al-MCM-41 do not work. Here, we observe that a relatively small amount of Al winds up as acidic acid sites for the Al-MCM-41 approach (the peak labeled tetrahedral Al), even for a much higher Al content in the synthesis (corresponding to a lower Si/Al) as compared to the Al-SBA-15. Direct 1H solid-state NMR observation of the Bronsted acid sites themselves also support this conclusion (results not shown).
Using a simple reactor constructed in-house by the students, gas-phase injections of a highly reactive olefin (2-butene) versus a much less reactive alkane (isobutane) were investigated, using the acidic mesoporous catalysts described in the preceding sections. Single pass conversions of 20 ml direct injections are reported in the following Table, recognizing that the carrier gas flow was 10-20 SCCM resulting in low conversion conditions for single-pass plug-flow conditions. The table indicates that both Al-MCM-41 and Al-SBA-15 effectively convert 2-butene at relatively low reaction conditions, as expected for such a highly reactive olefin. However, Al-SBA-15 still shows appreciable conversion of isobutane, while Al-MCM-41 does not. For reference, results for acidic HZSM-5 zeolite are also shown for isobutane conversion. Clearly, there is additional and more rigorous reactor work that could be done here, which will be pursued in future experiments. Again, these results indicate that Al-SBA-15 exhibits true Bronsted acid character, and is a viable candidate for large molecule reactions of the type currently inaccessible using traditional zeolites.
Table. Pulsed microreactor data for the conversion of isobutane and 2-butene over the indicated materials, at approximately equivalent loadings (# molecules per theoretical acid site). Note again the large disparity in Si/Al for the two mesoporous catalysts.
|
Mesoporous Materials
|
Isobutane
|
Butene
|
||
|
450°C
|
500°C
|
100°C
|
150°C
|
|
|
Amorphous SiO2 |
0.00 |
0.09 |
4.63 |
4.63 |
|
SBA-15 |
0.07 |
0.22 |
- |
- |
|
Al-MCM-41 (Si/Al=5) |
0.17 |
0.33 |
27.38 |
29.00 |
|
Al-SBA-15 (Si/Al=18) |
0.94 |
5.62 |
27.07 |
26.37 |
|
HZSM-5 (Si/Al = 13) |
|
20.5 |
|
|
While much of the work described above, arising from the most recent portion of the funding period, is not yet published, manuscript preparation is underway.