Reports: AC10
45215-AC10 Synthesis and Polymorphic Control for Visible Light Active TiO2 Nanoparticles
Kathleen Richardson, Clemson University
Research Activities
During the 3rd year of this project, research was focused on deactivation and regeneration of visible light active predominantly brookite titania nanoparticles.
The SACS technique, with N-methylpyrrolidone (NMP) as the
solvent, (U.S. Patent pending) developed by our research group has been used as
a post-synthesis treatment of WACS process [1-4].
The TiO2 powders were calcined in air at 200oC for 2
hours.
Characterizations
All samples were characterized by x-ray
diffraction (XRD), N2 physisorption, UV-Vis absorption
spectrophotometry, FT-IR, TGA, XPS, and TEM
The deactivation of polymorphic brookite titania was
evaluated by observing the photocatalytic degradation of MO under either UV
with wavelength of 365 nm, or VL irradiation of 560-612 nm. The experimental MO test and the calculated
MO degradation percent value, D, were obtained by the procedure found in the literatures [1-5].
Titania samples were
reused for four reaction cycle runs without any intermittent treatments in a
fresh MO solution. Sample IDs of used
TiO2 samples are composed of the fresh TiO2 sample ID, UV
or VL irradiation or dark, and # of cycle runs.
For example, the NMP-200 sample after the first run of MO degradation
under VL irradiation was called NMP-200-VL#1.
After the first reaction cycle,
the used sample was regenerated by several possible procedures, i.e., solvent washing
or recalcination at various temperatures.
The regeneration of the
catalyst was investigated by reusing 0.1 grams of a regenerated titania sample
in the second reaction cycle under the same condition as the first one.
Results
The physical properties of fresh
and used TiO2 samples and the reference VLP7000 are given in
Table 1. The reference Kronos
samples (VLP7000) are 100% anatase phase and have similar crystallite size to
our prepared titania. The surface areas
of as-prepared
titania samples are
approximately 1.8 times smaller than that of Kronos sample.
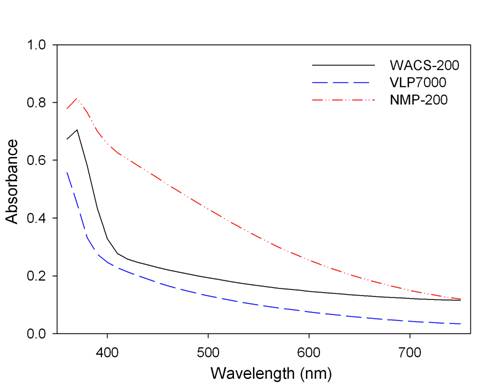
UV/Vis spectra of titania samples are given in Figure 1. The NMP-200 sample exhibited a shift of the absorption shoulders to the VL
region, compared to WACS-200 and VLP7000.
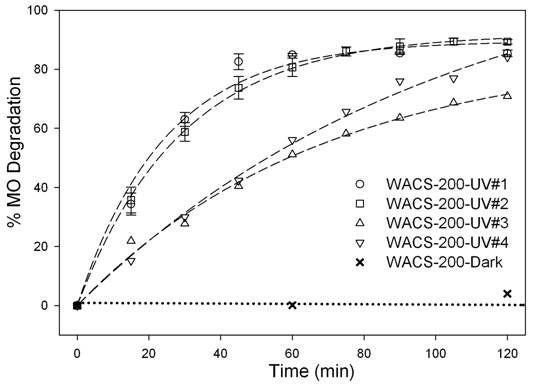
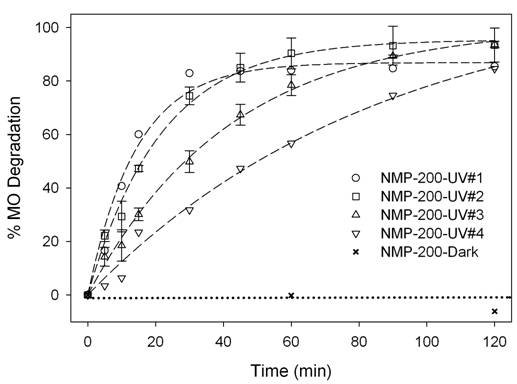
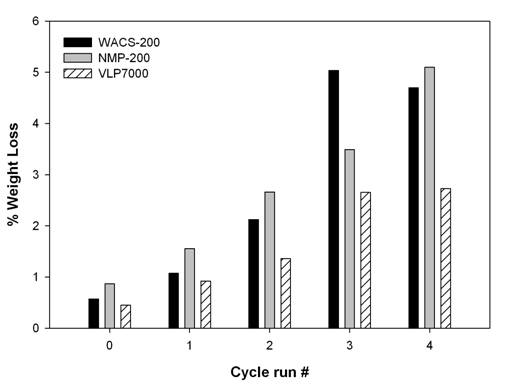
The stabilities of WACS-200
and NMP-200, determined by the MO degradation under UV irradiation, are
given in Figures 2(a) and 2(b), respectively.
MO with titania samples stirred in the dark was not degraded. The MO degradation by NMP-200 was 10 minutes
faster than by WACS-200, as expected, because of fewer lattice hydroxyls in NMP-200
sample [2, 3]. Both samples did not deactivate after the
first reaction cycle. After the second
cycle, however, PCA of both samples gradually decreased with the increase in
the testing time.
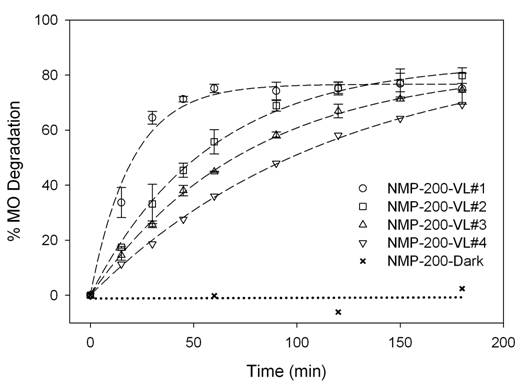
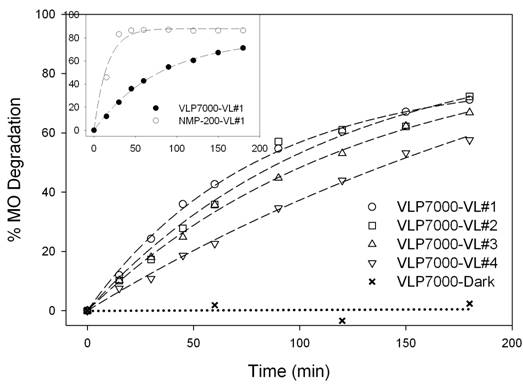
The deactivation of NMP-200 and VLP7000, evaluated by MO
degradation under VL irradiation, is reported in Figures 2(c) and 2(d),
respectively. The MO degradation rate by
NMP-200 exhibited approximately 3.3 times higher than that of VLP7000, as shown
in the inset of Figure 2(d), despite the much lower surface area of NMP-200 than that of VLP7000. This VLA superiority of NMP-200 must be due to the
presence of brookite phase with the significant proportion of anatase, as
reported in our previous paper [4], compared to 100% anatase
for VLP7000. It is also supported
by UV/Vis spectra in Figure 2(a) that NMP-200 can absorb more VL than
VLP7000. Nonetheless, both NMP-200 and
VLP7000 gradually deactivated with the increasing number of reaction cycles. The catalyst deactivation is possibly
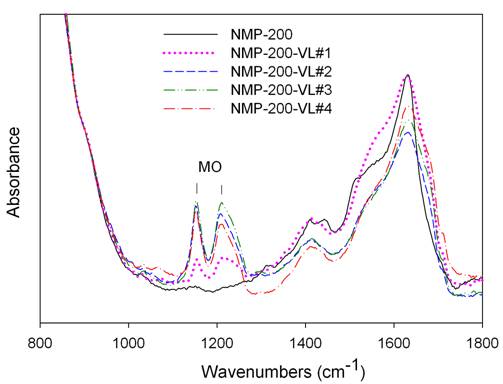
attributed to the deposition of the decomposed MO. The carbonaceous deposit, called coke [6-8],
was examined by FT-IR (Figures 3), TGA (Figure 4), and XPS.
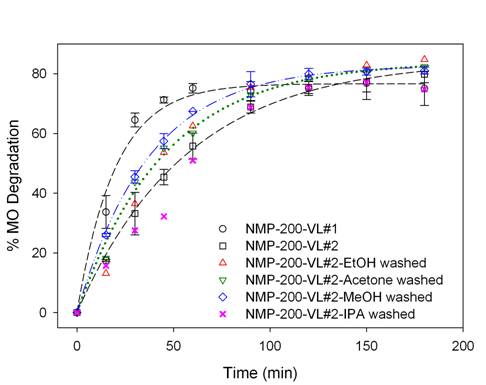
To study the catalyst
regeneration by solvent washing, NMP-200-VL#1 was washed with various solvents,
i.e., methanol (MeOH), ethanol (EtOH), isopropanol (IPA), or acetone. Figure 5 presents the regeneration of
NMP-200-VL#1 by washing evaluated by the MO degradation under VL
irradiation. Among the washing
procedures of the used samples, MeOH washing was shown to be the most effective
up to ~80% recovery of the fresh sample.
Table 1:
Sample ID
Crystallite size (nm)a
BET surface area (m²/g)b
Pore volume (cm³/g)b
Pore size average (Å)b
Anatase
Brookite
Rutile
WACS-200
5
9
11
169
0.1
25
WACS-200-UV#1
7
10
10
177
0.1
27
NMP-200
7
8
13
157
0.1
24
NMP-200-VL#1
7
9
11
220
0.1
25
NMP-200-VL#2
5
8
16
202
0.1
26
NMP-200-VL#3
6
7
12
175
0.1
27
NMP-200-VL#4
7
7
10
185
0.1
27
NMP-200-UV#1
6
7
12
206
0.1
25
VLP7000
7
-
-
290
0.4
56
VLP7000-VL#1
9
-
-
287
0.4
59
a Calculated from
XRD data using the Scherrer equation. Error of measurement = ±5%.
b Using N2
physisorption at -196°C. Error of measurement = ±10%.
Figure 1
Figure 2(a)
Figure 2(b)
Figure 2(c)
Figure 2(d)
Figure 3
Figure 4
Figure 5
Conclusions
The deactivation of WACS-200 and NMP-200 was evaluated by the
degradation of MO under either UV or VL irradiation for 4 reaction cycle runs
without intermittent treatments, and then compared to VLP7000. NMP-200 was found to be much more VLA than
VLP7000, although NMP-200 has 1.8 times smaller BET surface area than
VLP7000. This VLA superiority of NMP-200 is due to mixed
brookite and anatase titania phases, compared to pure anatase phase of VLP7000. However, PCA under VL of both NMP-200 and
VLP7000 gradually decreased with increasing the number of reaction cycles. The cause of the deactivation of the titania
samples in this study was identified as the deposition of the decomposed MO or
the carbonaceous deposit, called coke.
The regeneration of the used NMP-200 samples was also
investigated. Among the solvent washing
procedures for used NMP-200, MeOH washing was shown to be the most effective
with up to ~80% of the PCA recovery.