Reports: AC7
46833-AC7 1,3-Dipolar Cycloaddition for the Creation of Conjugated Polymers
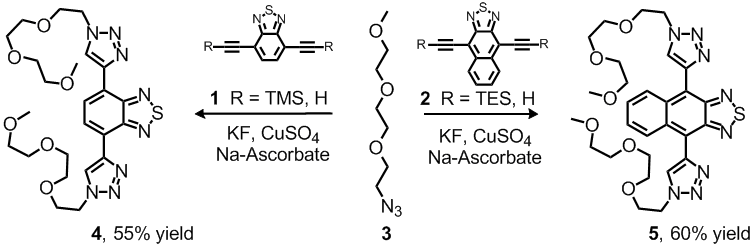
A less explored aspect of the triazole formation is their incorporation, into fluorophores; the triazole group could either work as an auxochromic group or a conjugative bridge between two chromophores or p-systems. Triazoles are quite powerful auxochromes, i.e., functional groups that red-shift the absorption and emission of acenothiadiazole-types. The novel water-soluble and fluorescent bis-cycloadducts 4 and 5 were prepared. These display binding pockets for metal cations. The oligoethylene azide 3 a) confers water solubility and b) is known for its biophobic properties, i.e., it suppresses non-specific interactions with proteins, etc.
Scheme 1. Synthesis of compounds 4 and 5.
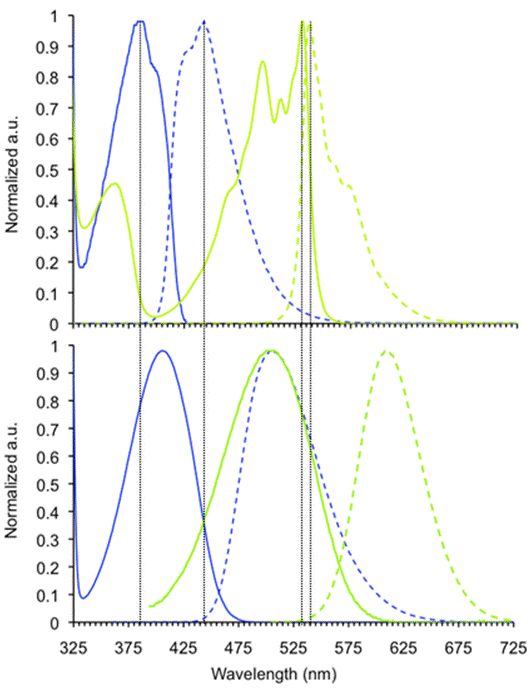
Figure 1. Absorption and emission spectra of 1 (blue), and 2 (green, both Top) and 4 (light blue) and 5 (gold, both Bottom) in dichloromethane. Solid lines depict absorption spectra while broken lines depict emission spectra. In the case of 1 and 2, the TIPS protected analogue was utilized due to its greater stability than the corresponding TMS or TES analogues.
Figure 1 shows the absorption and emission spectra of 1, 2, 4 and 5. Adduct 4 displays a quite robust quantum yield of 13% in water. The emissive lifetimes of 4 in DCM (14 ns) and in water (7 ns) solutions are unusually high. The larger congener 5 displays similarly large fluorescence lifetimes of 18 ns and 5 ns in DCM and water, respectively. The fluorescence lifetime of 4 is promising for potential applications as a bio-fluorophore. As complex biological matrices such as cells, serum, etc. are always fraught with background fluorescence with an emissive half life of around 3 ns, fluorophores such as 4 should shine if time-gated detection is used. Quite unusual is also that the emission and absorption wavelengths of 4 and 5 are not very solvent dependent; a slight blue-shift in the absorption features for both 4 and 5 when going from DCM into water are observed.
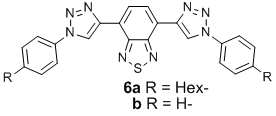
Surprising are the red-shifts in absorption and emission wavelengths of 4 and 5 in comparison to 1 and 2. Quantum chemical calculations (B3LYP 6-31G**//6-31G**) show that the HOMO and the LUMO positions are both raised, however the destabilization of the HOMO is more pronounced than the destabilization of the LUMO when going from alkyne to triazole. The absorption and emission profiles for 6a (DCM; lmax abs = 409 nm, lmax emission = 507 nm) are similar to those obtained for 4 and HOMO and LUMO of the model compound 6b at -5.69 eV and -2.66 eV. Consequently, while the triazole unit has a strong auxochromic effect, it is poor at transmitting electronic communication between two p-systems.
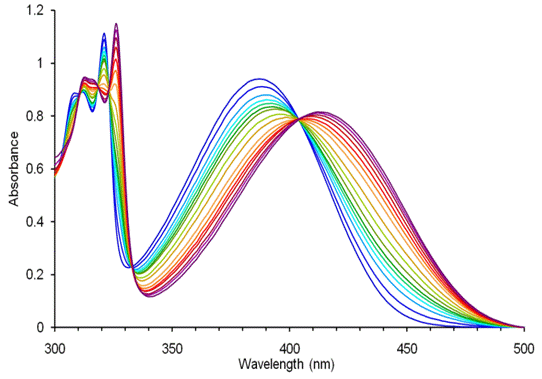
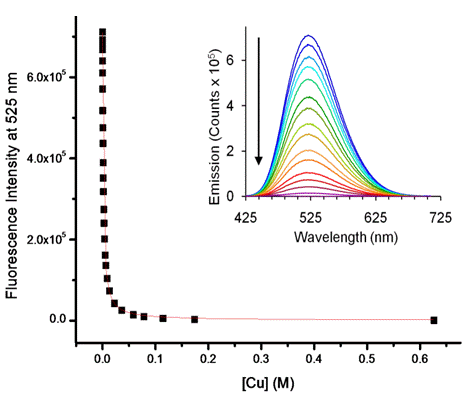
Figure 2. Representative absorption titration of 4 (blue trace; 151.5 mM) with CuSO4 in water. [Cu] ranges from 0-6.26 x 10-1 M (red trace 4-Cu2+ complex)(left). Emission data (black dots) of the titration of 4 (151.5 mM) with CuSO4 in water. Red line indicates the fitted equation used to determine binding constant. [Cu] ranges from 0-6.26 x 10-1 M. Inset: Representative emission titration of 4 with CuSO4 in water (right).
Adducts 4 and 5 display a binding pocket that should readily bind to metal analytes of appropriate charge and atomic radius. Upon the addition of Cu2+ or Ni2+ the charge transfer band in the absorption spectra redshifts (~20-30 nm) and the emission also quenches. Titrations of 4 and 5 with copper sulfate and 4 with nickel sulfate in water were performed to determine the strength of the binding.The binding of 4 to Ni(II) in water resulted in a binding constant of log K = 3.17 + 0.01. The binding constant for Cu(II) in water was slightly smaller in magnitude than that of nickel with log K = 2.70 + 0.01.
The binding constant for the binding of copper to 5 was determined to be log K = 2.71 + 0.03. This binding constant was nearly identical to that of 4 which demonstrated the independence of the binding upon the size of the aceno portion of the core. Interestingly, the necessity of a stoichiometric amount of copper in the synthesis of 4 and 5 can be attributed to this high binding constant as once the triazole group is formed, the effective concentration of free copper availible to catalyze the reaction is drastically reduced. Binding constants were also obtained from the deconvolution of the absorption spectra from the titration of the metal. Assuming a 1:1 complex agrees well with the obtained data. Apparently upon coordination to one copper ion, the second binding pocket becomes too electron poor to effectively bind another copper ion in aqueous solution.
Water soluble bistriazoles 4 and 5 and the model compound 6a suggest that the triazole-ring has a strong auxochromic effect and leads to red-shifted spectroscopic features for the connected arene in the 4-position, but at the same time is a poor electronic conduit, as the spectroscopic properties of 6a are almost identical to that of 4. The adducts 4 and 5 do not show large solvent dependencies of their spectroscopic properties, and 4 is surprisingly fluorescent in water binding both copper and nickel in aqueous solution. Overall, the 1,3-dipolar cycloaddition of alkynes to azides is a superb tool to prepare functional, metallo-responsive fluorophores.