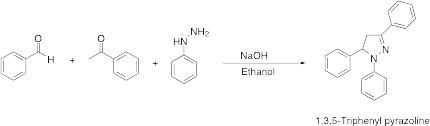Reports: B4
45137-B4 The Design and Study of Molecular-Scale Photonic Devices and Green Chemistry Approaches Towards Their Synthesis
We are interested in developing molecular-scale photoionic devices that can function as fluorescent switches with different cations. Most of these switches utilize photoinduced electron transfer (PET) to generate or quench fluorescence and are based on a chromophore-spacer-receptor architecture. Since many of these PET switches have tertiary nitrogen atoms as a part of the receptor, they generate fluorescent signals for various metal ions as well as protons. During the past year we have concentrated on several different aspects of our proposal including attempts to develop new PET systems, evaluating new chromophores to be used in PET systems and attempts to develop more efficient synthetic procedures for some PET systems.
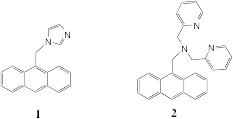
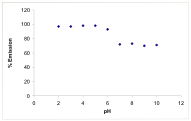
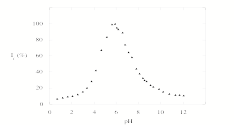
One of the new PET systems we studied during the last year was 1, which has an imidazole ring as the receptor. While 1 has been reported previously,1 imidazole rings have not been studied as proton receptors in PET systems. We have compared the fluorescence modulation of 1 with the off-on-off fluorescence switch, 2, that was developed in our lab in the past.2 Our preliminary studies show that the fluorescence of the anthracene ring in 1 is not completely quenched by the PET with the imidazole as we see with tertiary nitrogens in systems such as 2 (off-on component). The protonation of the imidazole leads to a fluorescence enhancement in 1 due to the quenching of the PET process. It is also interesting to note that while pyridine rings in PET sensors quench the fluorescence of anthracene chromophores (on-off component), the imidazole ring does not quench the fluorescence at low pH. The partial quenching of the anthracene fluorescence by the imidazole suggest that it is possible to develop new fluorescent PET switches that display more complex fluorescence modulation with pH.
Figure 1. Fluorescence intensity modulation of 1 [left] and 2 [right] (10-5M) with pH, in methanol/water (1:1). (λex = 350nm λem = 370-550nm). pH was adjusted by adding HCl & NaOH.
We have also been successful in developing a one-pot synthesis of the pyrazoline chromophore as part of our studies supported by this grant. Pyrazolines are commonly used as chromphores in PET systems and are usually prepared in two steps. We have been able to prepare 1,3,5-triphenyl pyrazoline in a one-pot synthesis by combining these two steps and we are currently in the process of optimizing this reaction.
The PRF grant provided financial support for our work including stipends for two undergraduate research students who worked with the PI during the summer.
1. Fan J.; Hanson, B. E. Inorg. Chem., 2005, 44, 6998.
2. de Silva, S. A.; Zavaleta, A.; Baron, D. E.; Allam, O.; Isidor, E. V.; Kashimura, N.; Percarpio, J. M. Tetrahedron Lett. 1997, 38, 2237.