Reports: B5
48436-B5 Interaction of Polyoxometallates with Organically-Modified Silica
I. Substrate Cleaning and Modification
In the past we have characterized our surfaces by depositing a drop of known
volume onto the horizontal substrate and measuring the diameter of the drop
when looking from above with a low-power microscope. Assuming that the drop
was small enough to be spherical (i.e., the shape was entirely owing to surface
forces and independent of gravity), the contact angle could be extracted from the
volume of the drop and the measured diameter of the surface. This method was
time consuming and difficult.
Using capital equipment funds from the grant, we acquired a commercial
contact angle goniometer. This drastically improved the reproducibility and speed
with which we could make contact angle measurements.
Several variations on our cleaning procedure were tested. Ultimately, we believe
that we have found a procedure that provides a reproducibly wettable
surface. First, we found that cleaning in Hellmanex (a commercial alkaline
detergent for cleaning glass cuvettes) was more effective at 67∘ C than at
60∘ C. Secondly, we found that the vacuum oven we had been using was
introducing significant contamination. Consequently, we now dry our
organically-modified surfaces under a stream of dry nitrogen, not in a vacuum
oven. Finally, we tested cleaning procedures from the literature which use
piranha solution or methanol/hydrochloric acid. These are less effective than
Hellmanex.
We also had a goal of improving our procedure for modifying our fused-silica
substrates with organo-silane molecules. After some investigation, we found that
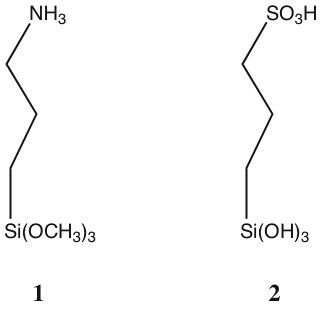
salinization was more reproducible when (3-aminopropyl)trimethoxysilane (1)
was exposed to the substrate from a methanol solution rather than a toluene
solution. Significant effort was also made to silanize the substrates from the vapor
phase, which may be important for forming chemical gradients on the
surface.
After measuring contact angles and adsorption to films exposed to
3-(trihydroxysilyl)-1-propanesulfonic acid (2), we now believe that this molecule
does not react at all with the silica substrate. We have begun preliminary work in
attempting to first modify the surface with (2-mercaptopropyl)trimethoxysilane,
followed by oxidation of the terminal mercapto group to sulfonic acid.This work is
very preliminary, and we do not yet have a satisfactory procedure for the
formation of sulfonic-acid modified surfaces.
Preliminary investigations were made into the pH dependence of the adsorption of
the polyoxometallate K6CoSiW11O39 to a fused-silica surface modified with
(3-aminopropyl)trimethoxysilane.
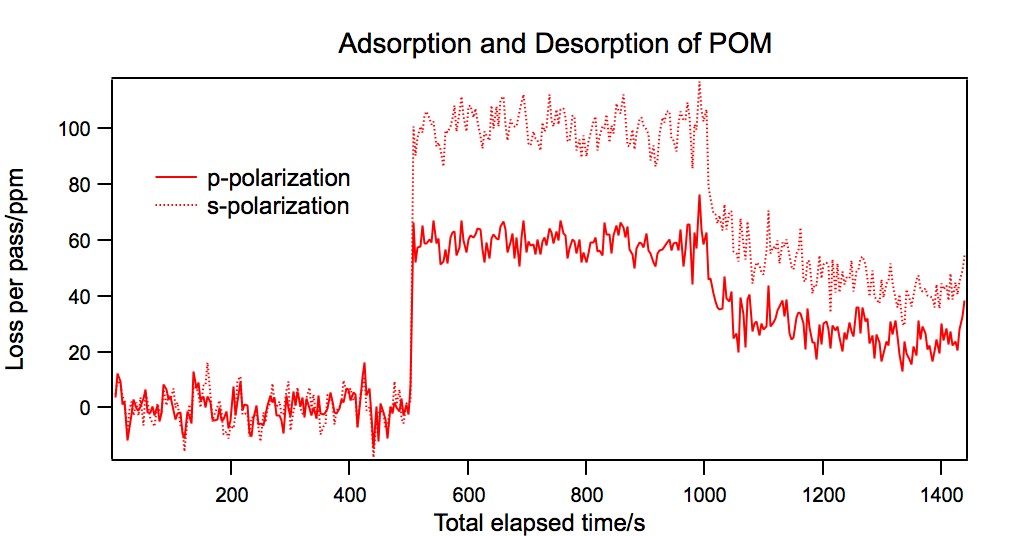
The data from a typical experiment are shown in Figure ??. Initially, there is
no loss in the cavity other than scatter and absorption losses from the mirrors and
prism, which are subtracted out. The POM is introduced to the surface, resulting
in a rapid increase in the per-pass loss. After 500 s the surface is again rinsed
with solvent. This results in a decrease in per-pass losses as POM desorbs.
Figure 1:
A fused silica surface modified with (1) was exposed to K6CoSiW11O39
at pH 2 and then rinsed. POM is introduced at 500 s and rinsed with solvent at
1000 s.
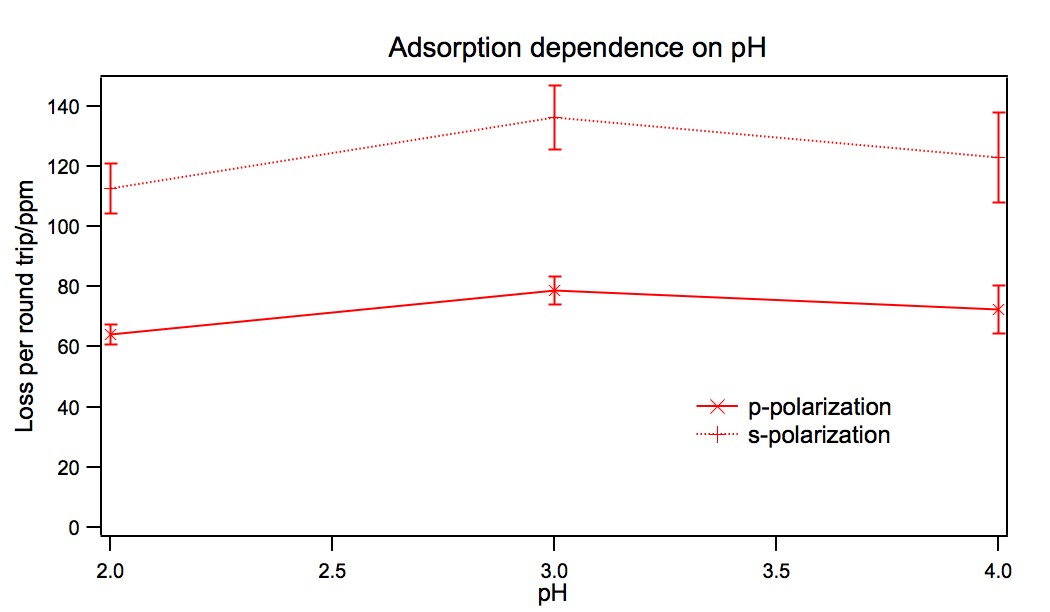
The results of several adsorption studies are shown in Figure 2. The
total amount of K6CoSiW11O39 is not strongly dependent on pH over this
range.
Figure 2:
The pH dependence of the adsorption of K6CoSiW11O39 to a
fused-silica surface modified with (1). Error bars are the standard deviation for
6–8 points.
Figure 2 includes the absorption for two different polarizations. The fact that
they are significantly different indicates the possibility of adsorbate orientation on
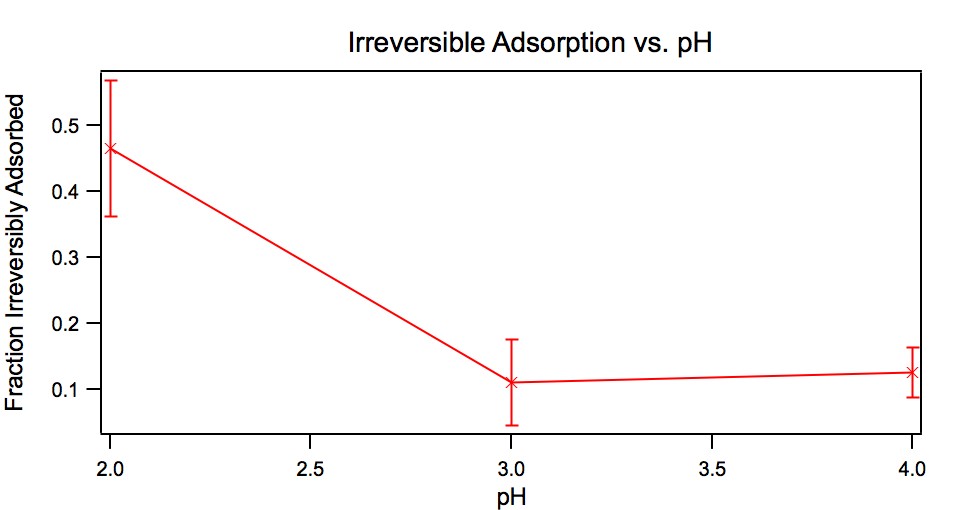
the surface. This will be the subject of future investigations. The reversibility of adsorption was also investigated as a function of pH. Figure 3
shows the fraction of the POM that was irreversibly adsorbed as a function of pH.
Approximately half of the originally adsorbed POM is found to be irreversibly
adsorbed at pH 2, while a smaller fraction is irreversible at the higher pHs. This
indicates that POM/aminosilane/silica film preparations are more stable at pH 2
than at pH 3 or 4.
Figure 3:
The pH dependence of the desorption of K6CoSiW11O39 from a
fused-silica surface modified with (1). The y axis is the fraction of POM remaining
after rinsing. Only p-polarization shown as s-polarization is almost identical.
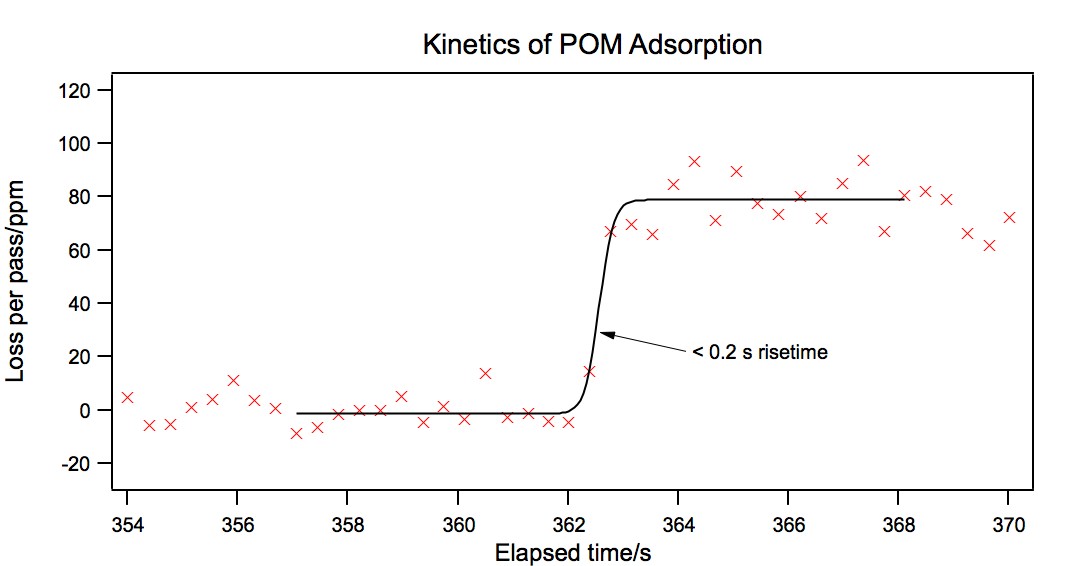
In order to measure the rate of adsorption with better time resolution, we
performed the adsorption measurement with no averaging. We were able to
acquire approximately 25 data point in 10 seconds.
The result is shown in Figure 4. As can be seen from the figure, the
adsorption is very rapid, and occurs on the same timescale as our experimental
repetition rate, or faster. Unfortunately, the rapidity of this process means that
we will not be able to fully characterize the kinetics of this system with our
present laser under these conditions. However, we believe that this is a significant
result in itself, and there is a chance that we will be able to study kinetics at lower
concentrations.
Figure 4:
Adsorption of K6CoSiW11O39 to a fused-silica surface modified with
(1). Data points were acquired as rapidly as transfer to the computer would allow.
We had not planned on accomplishing any gradient formation in the
first year of the grand period. However, we were able to do significant
preliminary work in this direction. Specifically, we began making gradients of
(3-aminopropyl)trimethoxysilane on fused silica substrates via two different
published methods: from solution and from a diffusing vapor. At present, it
appears as though the vapor deposition technique results in better surface
preparations.
Specifically, we are now able to produce gradients of (1) on a fused-silica prism
which have a contact angle of about 25∘ on one side, a gradually changing contact
angle over a distance of about 2 cm, and a contact angle of about 60∘ on
the other side. These are very promising thin films for future adsorption
studies.