Reports: B6
46291-B6 Condensed Phase Effects on the Structural Properties of Friedel-Crafts Intermediates: RF-BF3
Context: The main objective of this project is to characterize the structural properties of organofluoride – boron trifluoride complexes (RF'–BF3) in the gas-phase and in bulk, condensed-phase environments via computations and low-temperature infrared spectroscopy. These species are key intermediates in Friedel-Crafts reactions [1] - an important class of carbon-carbon forming processes that facilitate the conversion of petroleum feedstocks to commercially-viable compounds. One common example is the alkylation of benzene, viz.
The first step is the formation of an RF–BF3 intermediate [1], and it is known that the methyl complex is quite weak in the gas phase with an experimental B-F' distance of 2.42 [2], but reacts like an effective carbocation in solution [1]. Thus, its solution-phase reactivity can only be rationalized by invoking a substantial structural re-arrangement that results from interactions with the solvent.
Infrastructure: In the past year, we have purchased and assembled a 7-node, 56 CPU computational cluster, which has greatly expedited the computational part of this work. This acquisition was made possible not only with funds from this grant, but also startup funds from a recent hire, as well as generous hardware and administrative support from UWEC's Learning and Technology Services Department. We have been running almost all of our computational jobs on this system since last spring, and it has enabled us to use very high levels of theory.
N2–BH3,
a very interesting test system: Some time ago we discovered that the N2–BH3
complex had a very short B-N (< 1.6 ) according to density functional
theory, but the MP2 binding energy was only 7.1 kcal/mol. This past spring, we
resumed our work on this complex since we were need of a small test system to
run some performance benchmarks on our new cluster and experiment with Natural
Bond Orbital [3] and Atoms in Molecules [4] analyses of the bonding that will
be key for the RF'–BF3 studies. In the end, we conducted an
extensive study using both density functional theory (e.g. B3PW91, with basis
sets up to aug-cc-
pV5Z),
and post-HF calculations (e.g. CCSD, with basis sets up to 6-311+(2df,2pd)).
Select structural results are shown below, and it is apparent that there is
marginal agreement between DFT and post-HF methods, and moreover, the binding
energy is even less than that predicted by MP2, perhaps only a few kcal/mol. In
spite of this, NBO [3] and AIM [4] analyses indicate that the extent of charge
transfer (0.25 e- from N2 to BH3) and covalent
character in this B-N bond is comparable to systems with much larger binding
energies (OC–BH3 and H3N–BH3).
Thus, the complex has a very short, yet weak B-N dative bond. This work will
soon be in press in the Journal of Physical Chemistry A.
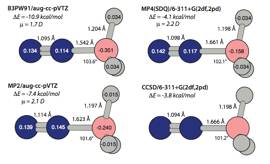
Gas-Phase Structures and Binding Energies of RF-BF3 Complexes
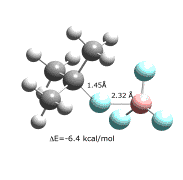
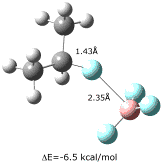
Last year we reported the gas-phase structure of CH3F–BF3, which had a calculated (X3LYP/aug-cc-pVTZ) B-F' distance of 2.41 , and an MP2/aug-cc-pVTZ binding energy of 3.6 kcal/mol. We now have obtained X3LYP structures for several conformers of (CH3)2CHF–BF3 and (CH3)3CF–BF3, of which the most stable are shown below. These complexes have B-F' distances that are only 0.05 shorter than the methyl complex, but binding energies that are more than 2 kcal/mol larger than for CH3F–BF3.
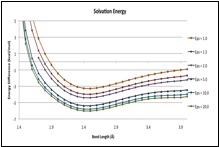
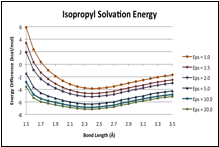
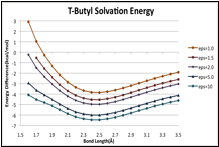
B-F' potentials: Gas phase and dielectric media
Calculated (X3LYP/aug-cc-pVTZ) B-F' potential curves for CH3F–BF3, (CH3)2CHF–BF3 and (CH3)3CF–BF3, are shown in the figures below. The gas-phase potentials (top traces in each curve) show no major peculiarities, but are rather soft long the inner wall, such that the energy only rises by about 2.5 kcal/mol over several tenths of an between the minima and the inner wall. The curves computed for dielectric media (via PCM [5]), do differ notably between the methyl complex and the two larger systems. As the dielectric increases, the potential of CH3CF–BF3 responds uniformly across all bond lengths, such that there is no change in shape, and no signs that a distinct structure with a shortened B-F' bond length is stabilized by the medium. For the larger complex however, the inner portion of the curve is preferentially stabilized. For (CH3)2HCF–BF3 and the potential at 1.7 is only about 1 kcal/mol above the minimum.
CH3F–BF3
(CH3)2HCF–BF3
(CH3)3CF–BF3
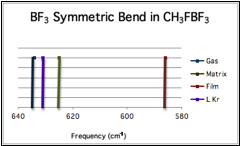
Frequencies: CH3F-BF3
At this point we have also collected IR spectra CH3F–BF3 and (CH3)2HCF–BF3, in both thin films and argon matrices. In the latter case, making spectral assignment has proved to be quite difficult. For the methyl complex, we have observed at least one key frequency shift - in the BF3 "Umbrella" mode, which red shifts in the early stages of B-F' bond contraction. Moreover, this shift is systematic across various media including our measured and calculated frequencies, as well as those previously measured in cryogenic liquids [6]. This depicts a subtle yet significant medium effect on the complex, which should be more extreme in the larger systems according to the computational results.
1. Olah, G.A. Friedel-Crafts and Related Reactions; Wiley/Interscience: New York, 1963.
2. Leopold, K.R.; Canagaratna, M.; Phillips, J.A. Accts. Chem. Res. 1997, 30, 57.
3. Reed, A.E.; Curtiss, L.A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
4. Bader, R.F.W. Atoms in Molecules - A Quantum Theory; Oxford University Press: Oxford, 1990.
5. Miertus, S.; Tomasi, J. Chem. Phys, 1982, 65, 239.
6. van der Veken, B.J.; Sluyts, E.J. J. Phys. Chem. A. 1997, 107, 9070.