Reports: AC10
47043-AC10 Side Chains with Incompatible Packing: A Strategy to Assemble Organic Semiconductors
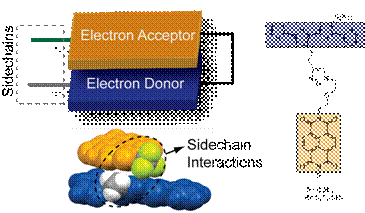
In organic-based photovoltaic (OPV) cells, it is a challenge to assemble p-conjugated semiconductors, which act as electron- and hole-conductors, into nanoscale structures with a heterojunction between the charge-carrier conductors for efficient charge separation and continuous phases of the charge-carrier conductors for high charge mobility. Why is it difficult to obtain segregated structure of hole-conducting and electron-conducting moieties? One reason is that electron conductors are electron deficient and hole conductors are electron rich; there is a natural tendency for electron-deficient compounds to mix with electron-rich compounds. Such mixed structures are extremely common both in small molecule and in macromolecular systems, and have been extensively studied in supramolecular chemistry. The key to avoid mixing is to destabilize interactions between electron-rich and electron-poor moieties and stabilize the interactions between electron-rich electron-rich and between electron-poor electron-poor moieties. If we can do that, then these semiconductors will spontaneously assemble into nanophase segregated structures with a domain of electron conductors, a domain of hole-conductors and an interfacial region between electron- and hole-conductor. Our approach involves appending side chains to electron-rich and electron-poor moieties such that side chains are immiscible. Based on chemical nature and packing propensities, we had identified various pairs of moieties that are immiscible. The archetypical example of such a pair is where one moiety is hydrophobic and another moiety is hydrophilic. Similarly, it well known that an aliphatic fluorocarbon will not form a co-crystal with an aliphatic hydrocarbon. Consequently, if a molecule has hydrocarbon and fluorocarbon sections, then in the solid state packing, these sections phase separate. Similar observations have been made in polymeric and dendritic systems. The PRF grant allowed my research group to establish a research program that probes the impact on sidechains on the packing of electron donors and electron acceptors.
Key Accomplishments at the End of Second Year.
The proposal had identified five research objectives. The current status for each these objectives is shown below
|
Specific Research Objectives (reproduced verbatim)
|
Current Status
|
|
To synthesize molecular dyads with terthiophenes or quaterthiophenes as the p-type semiconductor and naphthalenediimide as the n-type semiconductor
|
Accomplished and Publication in Progress
|
|
To synthesize semiconductor dyads with side chains that will have incompatible packing
|
Accomplished and Publication in Progress
|
|
To crystallize the dyads and study the solid state packing using single crystal X-ray crystallography
|
In Progress
|
|
For molecules that do not crystallize, characterize the morphology of the annealed thin films from these dyads using X-ray diffraction or scattering, electron microscopy and atomic force microscopy, and
|
Accomplished
|
|
To show that side chain pairs with incompatible packing can be used to create heterojunctions for photovoltaic cells
|
Accomplished
|
As mentioned in the Year 1 report, we have synthesized oligothiophenes and polythiphenes with side-chains bearing protected terminal alkynes and asymmetric NDIs bearing hydrocarbon or fluorocarbon side-chains. The dyads and the polymers were assembled via 1,3-dipolar cycloaddition commonly referred to as the click chemistry.
| Scheme 1: Dyads studied in year 2
|
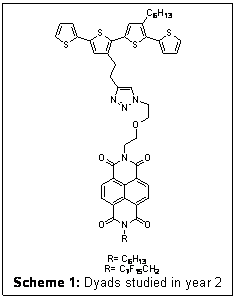 More information about the photophysical properties of the DBA molecules were
obtained from time-resolved photoluminescence (PL) measurements. We obtained
the PL dynamics of donors and the dyads (Scheme 1) measured at 500 nm, close to
the emission maximum of the donor molecules (PL dynamics at other wavelengths within
the donor emission spectrum show very similar decays) in collaboration with
Prof. Marc Achermann in Physics at UMass Amherst. Our comparison showed that
the dyad PL decay was faster than the donor PL dynamics. We determined decay
times of 460 ps for the donor (quaterthiphene) and 210 ps, and 230 ps for the dyads
bearing hydrocarbon and fluorocarbon side chains respectively.. From the decay
times we can determine the electron transfer rates of 2.2 for the fluorocarbon
and 2.6 ns-1 for the hydrocarbon and fluorocarbon chains, respectively.
The difference between the acceptors in the two dyads is small but has
consistently been measured. For the electron transfer efficiency η = 1
τD/τDBA we determine 54% and 50% for hydrocarbon
and fluorocarbon chains respectively. These studies clearly indicate that the
sidechains play a major role in the interaction between electron donor and electron
acceptor. At the present time, we are
investigating whether the sidechains impact the distance between the donors and
acceptors or the geometry of the donor-acceptor interaction or both (Figure 1). This work will be submitted for publication
at the end of this calendar year.
More information about the photophysical properties of the DBA molecules were
obtained from time-resolved photoluminescence (PL) measurements. We obtained
the PL dynamics of donors and the dyads (Scheme 1) measured at 500 nm, close to
the emission maximum of the donor molecules (PL dynamics at other wavelengths within
the donor emission spectrum show very similar decays) in collaboration with
Prof. Marc Achermann in Physics at UMass Amherst. Our comparison showed that
the dyad PL decay was faster than the donor PL dynamics. We determined decay
times of 460 ps for the donor (quaterthiphene) and 210 ps, and 230 ps for the dyads
bearing hydrocarbon and fluorocarbon side chains respectively.. From the decay
times we can determine the electron transfer rates of 2.2 for the fluorocarbon
and 2.6 ns-1 for the hydrocarbon and fluorocarbon chains, respectively.
The difference between the acceptors in the two dyads is small but has
consistently been measured. For the electron transfer efficiency η = 1
τD/τDBA we determine 54% and 50% for hydrocarbon
and fluorocarbon chains respectively. These studies clearly indicate that the
sidechains play a major role in the interaction between electron donor and electron
acceptor. At the present time, we are
investigating whether the sidechains impact the distance between the donors and
acceptors or the geometry of the donor-acceptor interaction or both (Figure 1). This work will be submitted for publication
at the end of this calendar year.
Figure 1: Illustration of the interaction between the electron donors and acceptors in a dyad in solution. A chemdraw rendition of the dyad is shown on the right. The conformation was calculated using a molecular mechanics forcefield.
With regard to polymers, a new monomer was developed for the postpolymerization functionalization of polythiphenes. The conditions for the copper-catalysed cycloaddition reaction were optimized because the standard condition of CuSO4/Na ascborate/water did not work for the polymers. UV-vis and CV measurements showed that the donors and acceptors behave independently when covalently attached in this fashion. The polymer work was published in the journal Macromolecules (Benanti, T. L.; Kalaydjian, A.; Venkataraman, D. "Protocols for Efficient Postpolymerization Functionalization of Regioregular Polythiophenes," Macromolecules 2008, 41, 8312-8315).
In the future, we are developing diblock polymers that have incompatible sidechains for photovoltaic applications.