Reports: AC4
47590-AC4 Design of New Radical Reactions: From Elusive 5-Endo-Dig Cyclization to Cascade Transformations
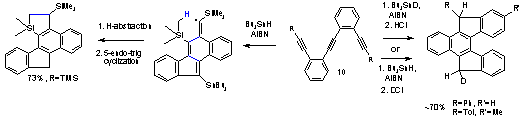
We, for the first time, offered theoretical analysis of a scarcely studied radical cyclization the 5-endo-dig process. Although this path is apparently favorable according to the Baldwin rules, only a few isolated examples of this reaction were reported, mostly in recent years. Unfortunately, most of these reports are speculative and, in some cases, are likely to proceed through alternative reaction cascades. Moreover, the majority of literature examples are relatively inefficient for reasons that were not clear before our study. Our work elucidated stereoelectronic, thermodynamic and polar contributions to the cyclization and extended these findings to a critical analysis of available experimental work. The general factors responsible for the efficiency of such processes were clearly identified and predictions about the experimental feasibility of 5-endo-dig radical cyclizations were made (JACS, 2005, 9534). Our experimental studies inspired by these computational predictions led to the discovery of the first efficient 5-endo-dig cyclization of a carbon-centered radical (JACS, 2008, 10984). We found that H-bonding interaction (estimated to be. Ca. 2 kcal/mo by NBO analysis) between the relatively acidic sp-C-H bond and one of oxygens from the Ts group is not only able to selectively accelerate the 5-endo-dig cyclization but can also control competition between the 5-endo and 4-exo modes. Our subsequent studies of scope and limitations of this effect already led to the discovery of several other, synthetically useful, 5-endo-dig ring closures of carbon-centered radicals.
In the second theoretical development, we analyzed the competition between two important radical cyclization processes 5-exo-dig and 6-endo dig cyclizations (JACS, 2005, 12583). These processes are used in organic synthesis and are implicated in the process of formation of polycyclic aromatic compounds and carbon nanostructures. When the acetylene moiety and the radical are connected through a saturated two-atom bridge, the 5-exo process is strongly favored kinetically. However, when the bridge is unsaturated, the 6-endo products are stabilized by aromaticity. Part of the product stabilization is transferred to the decrease in the 6-endo activation barrier rendering it kinetically competitive with the 5-exo-dig path. As a result, the 5-exo or 6-endo selectivity can be fine-tuned by seemingly subtle modifications in the structure of the starting materials. Our study determined the contributions of thermodynamic and strain effects to the 5-exo/6-endo competition, extended these findings to available experimental data and provided predictions to guide our experimental studies which led to the development of an efficient radical cascade providing polyclic systems representing the tip part of carbon nanotubes (JACS, 2008, 11535).
Ortho-effect in the Bergman cyclization: kinetics and development of new radical cascades: We applied steric and electronic effects of ortho substituents for efficient control of thermal Bergman cyclizations of benzannelated enediynes. Theory predicts that change in ortho substituents can lead to a nearly 2000-fold difference in the cyclization rate (250-fold greater range than for para substituents).
We confirmed these computational predictions with experiments and proved that steric and electronic effects of ortho substituents can be used for efficient control of thermal Bergman cyclizations of benzannelated enediynes.
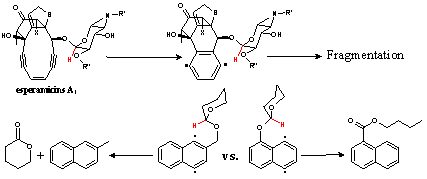
Interception of p-benzynes by intramolecular H-abstraction increases the apparent reaction rate by rendering the cyclization step effectively irreversible. From a chemical perspective, such interception is interesting because it produces a new, more persistent diradical which is not capable of deactivation through the retro-Bergman opening. In addition, intramolecular H-abstraction in OMe-substituted p-benzynes followed by domino radical cyclizations can be used to transpose radical centers in p-benzyne and create new types of diradical species. Interestingly, the same step is possible in natural enediyne antiobiotics of calicheamicin and esperamicin families where a OCHR substituent is similarly positioned relative to the p-benzyne intermediate. We found that such hydrogen abstraction can rationalize the so far unexplained fragmentation of esperamycin A upon its activation towards cycloaromatization. In addition, an interesting radical rearrangement which transposes oxygen and carbon atoms attached to an aromatic ring was discovered experimentally (JACS, 2009, submitted).