Reports: GB1
46492-GB1 Catalytic Asymmetric Methods for the Conversion of Petroleum Products Derived from Ethylene and Propylene into Chiral N-Heterocycles: the Preparation of Chiral Pyrrolidines and 1,2,3,4-Tetrahydroquinolines
A Tandem Michael-Aldol Reaction for the Preparation of Dihydro-quinolines. 1,2,3,4-Tetrahydroquinolines and dihydroquinolines are common structural motifs in many biologically-active molecules, many of which have industrial and medicinal applications. Due to the importance of this compound class, the development of synthetic routes for the preparation of these compounds is of great synthetic interest.
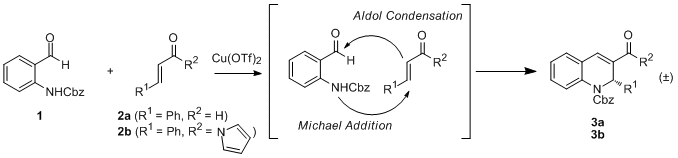
Our initial route to access these compounds was to employ a,b-unsaturated aldehydes with proline-based iminium ion catalysis in a tandem Michael-aldol reaction. Unfortunately, a recent report by Wang and coworkers, in which the same catalyst and substrate scope were utilized, was published during our investigations of this route. As a result, we focused our subsequent efforts on the study of some unusual reactivity that we had observed during our related studies of the metal-catalyzed version of this reaction. Through considerable metal screening, we determined that copper(II) triflate (10 mol%) could catalyze the tandem Michael-aldol reaction of cinnamaldehyde 2a with a 2-N-Cbz-protected benzaldehyde 1, albeit in low conversion (19% after 48 h; Scheme 1). No conversion was observed when triflic acid, a compound commonly present in metal triflate reactions, was employed as the reaction catalyst, indicating that catalysis is metal-based. The use of a strongly-donating solvent, such as acetonitrile, proved crucial to reactivity; no conversion was observed in the presence of a noncoordinating solvent.
Scheme 1. Tandem Michael-aldol condensation.
Optimization of the Michael acceptor led us to investigate a,b-unsaturated N-acyl pyrrole 2b, which afforded the desired Michael-aldol product in 43% conversion after 48 h. Unexpectedly, the corresponding phenyl ketonewhich has previously been used interchangeably with N-acyl pyrroles in organometallic reactionsafforded no reaction. In addition, the interchange of Cbz for tert-butoxycarbonyl (Boc) as the protecting group on 1 also led to negligible product formation, largely due to decomposition. The best results with this catalyst loading (93% conversion after 48 h) were obtained when the reaction was conducted at reflux. Interestingly, negligible reactivity was observed with other metal triflate salts commonly used in Michael additions.
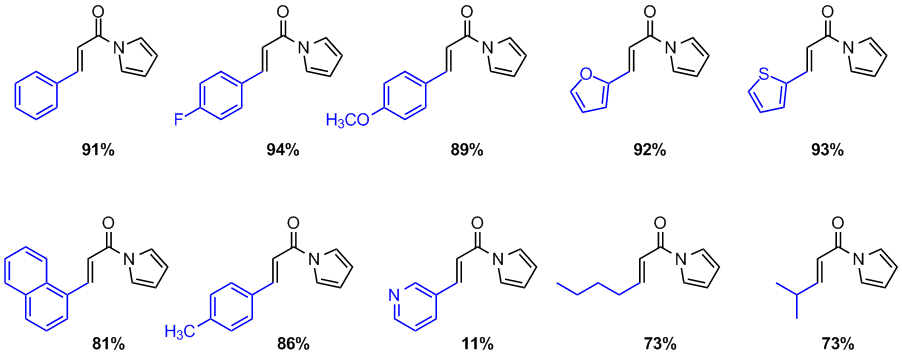
To date, these reactions have proven to be remarkably clean, with GC conversion data correlating with isolated yield. Reaction scope has been extended to include several aromatic and aliphatic substrates (Figure 1). To our knowledge, the unusual selectivity of this reaction for N-acyl pyrroles in the presence of carbamate-protected nucleophiles is unprecedented, and stands in marked contrast to previous reports. Product characterization and computational studies into the selectivity of this reaction are currently underway. We seek to submit this work for publication in Tetrahedron.
Figure 1. Scope of the copper-catalyzed tandem Michael-aldol reaction with 1.
It is important to note that research
on this project has been conducted entirely by undergraduates: lead discovery of
the copper triflate system was performed by Claire Knezevic (Scripps '08); substrate
scope was investigated by Anna Wagner (CMC '09).
Electron-Deficient Biphenyldiol Catalysts for Asymmetric Catalysis.
While investigating potential catalysts for the tandem-Michael aldol reaction, members of our group began investigating possible Brønsted acid catalysts. The Brønsted acid-catalyzed activation of carbonyls and imines to nucleophilic attack is one of the most ubiquitous synthetic methods used in organic chemistry; however, it has not been until recently that the use of chiral Brønsted acids to catalyze asymmetric reactions has been actively investigated. Of these, the application of relatively strong protic acids, such as chiral phosphoric acids, are amongst the most recent, with the majority of reports occurring within the past five years.
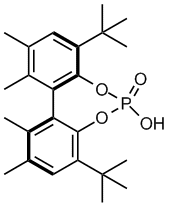
Figure 2. Phosphoric acid 3.
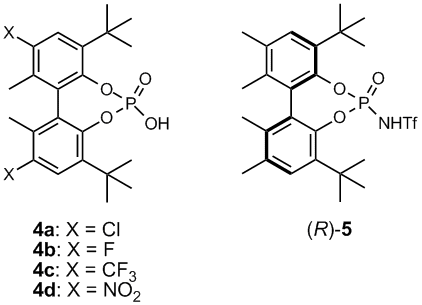
Seeking an alternative catalyst framework, we became intrigued with the chiral biphenyl diol phosphoric acid 3 (Figure 2), originally reported by Schrock and Hoveyda as a ligand precursor in the preparation of enantioselective olefin metathesis catalysts. In comparison to the previously reported binaphthol-based catalysts, acid 3 retains the desired design element of bulky 3'3-disubstitution in the form of tert-butyl groups, and the parent diol possesses chirality due to atropisomerism. Despite these design attributes, acid 3 lacks the electron-deficient substitution known to be beneficial to asymmetric catalysis. With this in mind, we sought to prepare catalyst family 4 (Figure 3), where X represents an electron-deficient moiety, such as chloro, fluoro, trifluoromethyl, or nitro. Ultimately, we were able to a facile, inexpensive synthetic route to the preparation of (S)-4a (5 steps, 76% of theoretical yield, accounting for resolution).
Figure 3. New phosphoric acid and thiophosphoramide catalysts.
In addition to 4a, we have also prepared biphenyl-based thiophosphoramide catalyst (R)-5. Chiral, binaphthol-derived thiophosphoramide catalysts were recently reported by Yamamoto and coworkers to display enhanced reaction rates and asymmetric induction relative to their corresponding phosphoric acids. The performance of these catalysts in both the hydrophosphonylation of imines and the Friedel-Crafts alkylation of indole has been studied. When employed in the Friedel-Crafts addition indole to chalcone, the chloro-substituted phosphoric acid was found to rival the best Brønsted acid catalysts reported to date (57% ee). This work is currently being written up for publication to Adv. Synth. Catal. for submission in 10/09.
As with the Michael-aldol
project, this work conducted by undergraduates: Elisa Gutierrez (CMC '09), Eric
Moorhead (CMC '08), Vivian Lin (Scripps '09), Eva Smith (Scripps '09), Laura
Ackerman (CMC '09), Claire Knezevic (Scripps '08), and *Note: CMC =