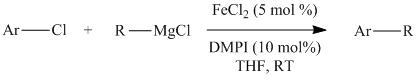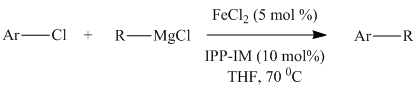Reports: B1
48541-B1 N-Heterocyclic Carbenes in Iron-Catalyzed Cross-Coupling Reactions
A summary of the work that was proposed to take place this past year is given below:
1) Synthesize a series of discrete dichlorobisimidazol-2-ylidineiron(II) and dichlorobisimidazolin-2-ylideneiron(II) complexes with varying N-alkyl substituents for screening in the cross-coupling of 2-chloropyridine and cyclohexylmagnesium chloride.
2) Optimize the conditions for the cross-coupling of 2-chloropyridine and cyclohexylmagnesium chloride using the best catalyst.
3) Test the cross-coupling of other aryl chlorides with cyclohexylmagnesium chloride and test the aryl chlorides with other secondary alkyl Grignards
4) Draft a communication to a peer reviewed journal.
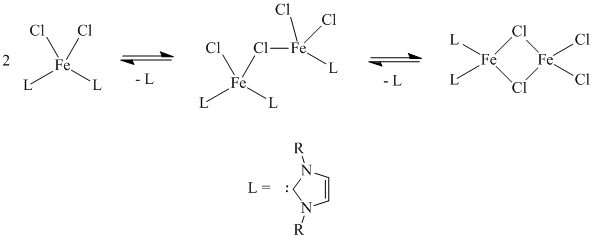
The synthesis of the discrete iron(II) N-heterocyclic carbene complexes ended up being a tremendous challenge, as once formed, they rapidly decomposed to form highly insoluble material. Although it has not been verified, it seems as though the process shown below was taking place based on elemental analysis of the products formed.
Due to the dramatic insolubility of the compounds produced, we were not able to fully characterize them. Also, given that the trial reactions showed that cross-coupling could be accomplished by forming the catalyst in situ we decided to stop trying to synthesize the iron complexes and focused instead on investigating the cross-coupling reactions in which the catalyst was formed in situ.
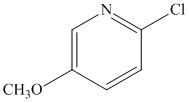
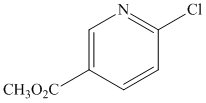
Although the N-heterocyclic carbenes were proving to be effective ligands for the cross-coupling of aryl chlorides with secondary alkyl Grignards in a few test examples, we could not necessarily attribute their effectiveness to their ability to act as p-acids in these systems. This is particularly true considering that there is still debate in the literature concerning the s-donor versus p-acid properties of these ligands. For this reason, I decided to test an isonitrile as a ligand in the cross-coupling of 2-chloropyridine and cyclohexylmagnesium chloride since they are known to be p-acidic and a variety of iron isonitrile complexes are known. The isonitrile chosen was 2,6-dimethylphenylisocyanide (DMPI) as it is a nice solid and has some steric bulk. Gratifyingly the reaction worked very well in that 2-chloropyridine was coupled quantitatively with cyclohexylmagnesium chloride in less than 20 minutes using 5 mol % FeCl2 and 10 mol % DMPI in THF at room temperature. We then tested a series of substituted chloropyridines and secondary alkyl Grignards in the cross-coupling using FeCl2 and DMPI. The results are shown below in Table 1.
Table 1. Cross-Couplings Using Isonitrile Ligand
|
Entry |
Ar-Cl |
R (equivalence) |
Time |
% Yielda (B:L)b |
|
1 |
cyclohexyl (2) |
20 min |
99 |
|
|
2 |
isopropyl (3) |
20 min |
48 (47:1) |
|
|
3 |
isopropyl (6) |
20 min |
90 (89:1) |
|
|
4 |
sec-butyl (6) |
24 hr |
75 (36:1) |
|
|
5 |
cyclohexyl (2) |
20 min |
99 |
|
|
6 |
isopropyl (2) |
20 min |
99 (48:1) |
|
|
7 |
sec-butyl (2) |
20 min |
99 (48:1) |
|
|
8 |
cyclohexyl (2) |
1 hr |
96 |
|
|
9 |
isopropyl (6) |
20 min |
89 (29:1) |
|
|
10 |
sec-butyl (6) |
24 hr |
76 (12:1) |
|
|
11 |
cyclohexyl (1.5) |
20 min |
82c |
|
|
12 |
isopropyl (1.5) |
20 min |
89c (88:1) |
|
|
13 |
sec-butyl (1.5) |
20 min |
84c (83:1) |
|
|
14 |
cyclohexyl (2) |
20 min |
99 |
|
|
15 |
isopropyl (2) |
20 min |
99d |
|
|
16 |
sec-butyl (2) |
20 min |
99d |
|
|
17 |
cyclohexyl (2) |
20 min |
97 |
|
|
18 |
isopropyl (2) |
20 min |
72e (8:1) |
|
|
19 |
sec-butyl (2) |
20 min |
29e (13:1) |
a) yield determined by GC-MS. b)branched to linear ratio. c) some ketone due to Grignard addition
to the ester was observed. d) no linear isomer observed. e) quinoline was observed.
At least 14 of the compounds reported in the table above have never been reported before. Therefore, a detailed characterization of each is required. When this is finished, the work will be reported in the literature.
While performing the cross-couplings using the isonitrile ligand, we were able to synthesize cis-tetrakis(2,6-dimethylphenyl isocyanide)dichloroiron(II) and we obtained x-ray quality crystals such that its structure was solved. Dr Kraig Wheeler at Eastern Illinois University solved the crystal structure, which turned out to be interesting. It was the first crystal structure of a cis-tatrakis(isocyanide)dihaloiron(II) complex and the isocyanide ligands trans to chloride were found to participate in back bonding while those cis to chloride did not. This prompted me to draft and submit a paper to the Journal of Chemical Crystallography.
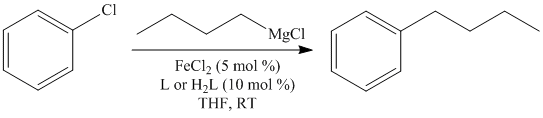
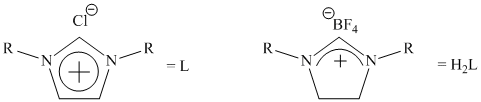
We also began an investigation into the use of N-heterocyclic carbenes as proposed, but we decided to focus on unactivated aryl chlorides with primary and secondary alkyl Grignards, as these substrates have not been reported at all using iron and are still challenging using the best palladium catalysts. In our test reaction we investigated the use of different carbene precursors in the iron-catalyzed cross-coupling of chlorobenzene and butylmagnesium chloride as shown below.
The R groups that we screened included mesityl, 2,6-diisopropylphenyl, and isopropyl. The imidazolium salt (L) with the 2,6-diisopropylphenyl substituents (IPP-IM) was found to be the most effective ligand. This ligand was then screened in the cross-couplings of three different primary alkyl Grignards as shown in the table below.
Table 2: Cross-Couplings of Unactivated Aryl Chlorides using N-Heterocyclic Carbene Ligands
|
Entry |
Ar-Cl |
R (equivalence) |
Time |
% Yielda |
|
1 |
butyl (6) |
1.5 hr |
99 |
|
|
2 |
isobutyl (6) |
1 hr |
99 |
|
|
3 |
propyl (6) |
2 hr |
96 |
|
|
5 |
butyl (6) |
1 hr |
87 |
|
|
6 |
isobutyl (6) |
2 hr |
96 |
|
|
7 |
propyl (6) |
3 hr |
99 |
|
|
8 |
butyl (6) |
3 hr |
87 |
|
|
9 |
isobutyl (6) |
3 hr |
99 |
|
|
10 |
propyl (6) |
3 hr |
81 |
a) Yield determined by GC-MS
The yields reported in Table 2 are not necessarily accurate as they were calculated by taking the peak area ratios of the starting materials and products. We have, however, run these reactions with naphthalene as an internal standard and are currently running the standards necessary to calculate actual yields. It is worth reporting that sec-butylmagnesium chloride was also shown to couple with chlorobenzene giving an overall yield of 76% with a 5:1 branched to linear ratio using similar conditions.
In conclusion, we have demonstrated the use of p-acidic ligands in the iron-catalyzed cross-coupling of aryl chlorides with alkyl Grignards. A system was developed for the use of secondary alkyl Grignards, which has not been developed to efficiency before. Also, the first iron-catalyzed cross-coupling of unactivated aryl chlorides has been developed.