Reports: B1
43717-B1 Porphyrin Arrays as Light Harvesting Compounds Formed from Porphyrin Modules and Metal Linkers
Our research program is focusing on the construction of porphyrin arrays built around metal centers through non–covalent interactions between the porphyrin and metals. Towards this end, we have been synthesizing porphyrins with heterocyclic substituents such an imidazole and an imidazolium substitute porphyrins as well as pyridyl porphyrins attached to osmium(II) metal centers.
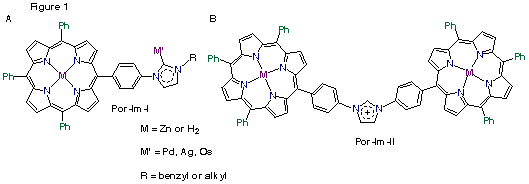
Imidazoles exhibit interesting metal binding properties, as they can bind through the nitrogen in the ring, or by forming a carbene complex. The imidazole porphyrin compounds were synthesized by two different approaches; in the first method, used for Por-Im-I, Figure 1a, the imidazole porphyrin was synthesized directly from imidazole benzaldehyde and the imidazolium was formed by the addition of the an alkyl halide or a benzyl halide. The second approach for Por-Im-II, Figure 1b, involves the synthesis of the imidazole ring between the two porphyrins. This compound exhibits unusual C–H hydrogen bonding as the acidity of the proton between the nitrogen atoms on the imidazole ring is enhanced by the charge on the ring. We have begun to investigate the binding of metal centers to the imidazolium carbene formed from Por-Im-I.
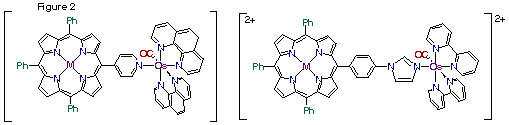
A second project involves the formation of a series of porphyrin-pyridyl-osmium compounds (Figure 2). The porphyrin-pyridyl-osmium compounds have been shown the to conduct light induced electron transfer and energy transfer reactions, depending on the presence of zinc(II) metal ions in the porphyrin. The current project extends this research by changing the substituents on the osmium metal ion from bipyridine to phenanthroline and substituted bipyridines and phenanthrolines and then studying the effect this has on the electron and energy transfer reactions. In addition, we are developing methods to attach the imidazole porphyrins to the osmium metal center through the nitrogen on the imidazole.
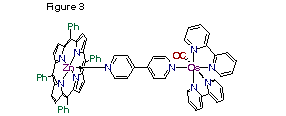
Finally, we have modified the attachment of the osmium moiety to the porphyrin by utilizing a linker with two Lewis bases, such as a 4,4'-bipyridine molecule, coordinate to the osmium on one side and a zinc metal ion inside of the porphyrin on the other. By changing the length of the linker we can investigate the interactions between the two molecules. We have shown that the attachment of the osmium moiety to the bipyridine does not affect the binding of the pyridine to the zinc metal ion.