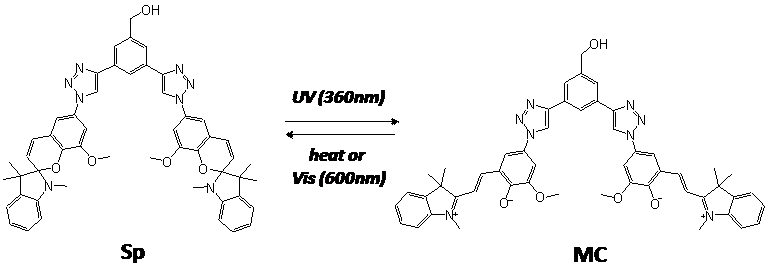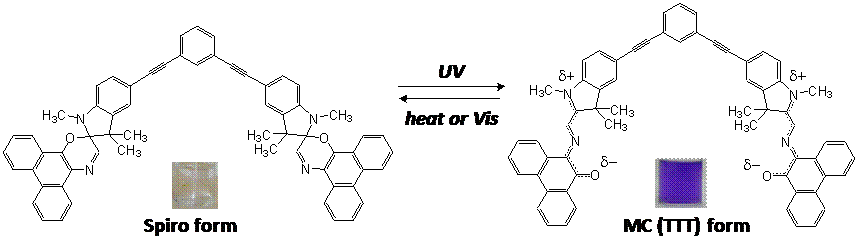Reports: GB7
46386-GB7 Design, Synthesis, and Photochromism Studies of Photo-Switchable Bispiropyran Polymers
The goal of this project was to develop the synthetic method and to understand fundamental properties of photo-switchable smart polymer systems that possess potential for site recognition of specific biomolecules and sensors of organic/inorganic compounds. Incorporation of unique shaped photochromic dye into polymers will enable construction of various self-assembly structures for new class of photo-responsive materials.
Spiropyrans are a class of photochromic compounds whose molecular structures are alterable upon exposure to UV/ visible light or changes in temperature. The typical reaction of spiropyran is the conversion between the non-polar Sp form with the polar MC forms. We are particularly interested in remarkable changes in polarity and structure (specifically, planarity) between Sp and MC forms. To effectively utilize these properties, we design a functional polymer having unique spiropyran dimer compound at the terminal. The polar face of the MC form dimer strongly attracts ionic species which can be released through visible light due to the transformation into the non-polar Sp form. The aggregates of the bispiropyran-polymer are more attractive since MC to Sp transformation destroys the planarity of the molecules, which will disallow the self-assembly structure of polymers. In this project, two models of bispiro compound-polymer conjugates are designed and synthesized (Figure 1). The self-assembly structures by phase separation of polymers and ionic interaction of bispiro MC forms will be studied using variety of techniques such as NMR, IR, AFM, TEM, and UV-vis. The fundamental photochromism study will disclose potential functions of novel bispiro polymer systems as smart molecular machine.
(a)
(b)
Figure 1. Photoreactions of BIPSD (a) and SPOD (b).
In the first funded year, we have devoted to synthesize two different photochromic dimers, 3,5-bis(1-(8-methoxy-1',3',3'-trimethylspiro[chromene-2,2'-indoline]-6-yl)-1H-1,2,3-triazol-4-yl)phenylmethanol (BIPSD) and 3,5-bis(1,3,3-trimethylspiro[indoline-2,2'-phenanthrooxazine]-5-yl)ethynylbenzylalcohol (SPOD).
SPOD synthesis: In addition to BIPSD, the new spirooxazine dimer, SOPD has been successfully prepared by multi-step synthesis route. The reactions include; Fisher Indol Formation from p-iodophenylhydrazine and 2-butanone, Sonogashira Coupling of iodoindole and 3,5-diethynylbenzylalcohol, and finally spiro-coupling reaction of the methylated indole dimer and 9-nitroso-10-phenanthrol. The Sonogashira reaction was a key to the entire synthesis scheme, and we optimized the reaction conditions and purification method.
Photochromism studies: Preliminary tests of photo reaction for BIPSD and its polymer systems have been performed using medium pressure mercury lamp and UV-vis spectroscopy. Visible light and heat treatments have also been examined and optimized for MC to Sp form conversion.
In the second funding year, detailed photochromism and binding studies on SPOD have been performed. Polymer-spiro dimer synthesis and analysis of photo-properties, as well as surface functionalization by photoswitch dimers have been studied.
Photo- and solvatochromism, and binding studies: It was found that two dimers, BIPSD and SPOD exhibited contrasting photo and solvatochromism from the kinetics study of thermal closure of spiropyran dimer in different solvents. A zwitterions form of the MC form of BIPSD was polar, thermal closing was slow especially in polar solvents, showed negative solvatochromism, and the MC form aggregated. In contrast, SPOD showed very fast thermal closing particularly in polar solvents, had positive solvatochromism, and fatigue resistance and superior photo repeatability between Sp and MC forms were observed. These results indicated that the MC form of SPOD exists in quinoidal form. The specific binding affinity of SPOD-MC form towards a palladium catalyst, Pd(PPh3)2Cl2 was confirmed.
BIPSD-polymer and SPOD-polymer synthesis: Biodegradable polymers, poly(L-lactide) (PLLA) and poly(e-caplolactone) (PCL), and the amphiphilic di- and triblock copolymers, poly(L-lactide)-b-poly(ethylene glycol), (PLLA-PEG and PLLA-PEG-PLLA), were designed to have BIPSD or SPOD on their terminals using a variety of polymerization/ coupling methods. Polymer-spiro dimer conjugates formed micelle structures in selective solvents and exhibited specific photochromism for each dimer and solvent, that indicated potential use of these polymers as photo-controlled delivery carriers.
Surface functionalization: A variety of surfaces such as glass, silicon, and polymer beads were functionalized by SPOD. Successful immobilization was confirmed and photoswitch surface was established.
Future developments of this project are to further investigate the self-assembled nanostructures and photoproperties of polymer-spiro dimer conjugates.
Impact on PI and students: A graduate student, Davita Watkins has been supported by this PRF grant for her summer stipend for two years. She has learned advanced organic synthesis skills through the research and made a significant progress on this project. Two undergraduates are also involved in this project. They have worked during summer with university scholarships. A postdoctoral associate, Satish Kumar has joined in our group in 2008 supported by our department. He has extensive experience on photochromics synthesis and photochemistry, which has accelerated this PRF project in the second year.
For the PI's career development, this PRF support was significant. Since she has moved from Boise State University to the University of Memphis in 2007, to continue and achieve her research progress during laboratory start-up couldn't have been smoothly done without the PRF fund. The promising results we have made by this grant will definitely become a big source for the large grants and publications.