Reports: G5
48308-G5 Superlattices of Perovskite Structured Materials for Solid Oxide Fuel Cells
The objective of this proposal is to gain an understanding of surface and interfacial phenomena in thin films and superlattices of perovskite structured materials for the improved performance of intermediate temperature solid oxide fuel cells (SOFC's). Perovskite structured materials are chosen due to the possibility of tuning their properties through chemical and structural parameters and the ability to meet stringent mechanical, electrical, electrochemical, and chemical stability requirements associated with the operation of SOFC's. La1-xSrxMO3 is the material of choice for cathode materials for high temperature SOFC's, however, the lack of oxygen vacancies leads to nearly exclusive electronic conduction and unacceptable performance at intermediate operating temperatures. In response, La1-xSrxMO3 with different transition metal cations (M=Fe, Co, and Ni) and their solid solutions have been investigated to achieve mixed ionic and electronic conductivity, good catalytic activity, good surface exchange, and high bulk diffusion. As an alternative to the solid solution, we study superlattices consisting of alternating perovskite layers to understand the coupling of materials properties at and across interfaces, particularly as the superlattice period decreases below characteristic lengths e.g. width of space charge regions for electronic and ionic conductivity.
Takamura has installed a state-of-the-art pulsed laser deposition (PLD) system at UC Davis capable of growing dense, epitaxial thin films and superlattice structures. A reflection high energy electron diffraction system is undergoing commissioning and will provide the monitoring of the growth mode and growth rates. Technical assistance and access to additional growth capabilities are provided by Dr. Hans Christian at the Center for Nanophase Materials Science at Oak Ridge National Laboratory. We have grown superlattices of La0.7Sr0.3MO3 where M= Mn, Fe, and Co (LSMO, LSFO, and LSCO) on SrTiO3 substrates. The notation for the superlattices consists of [# unit cells LSCO/LSMO ][# unit cells LSFO] # of repeats. For comparison, epitaxial films of LSMO, LSFO, LSCO, and chemically equivalent solid solutions, La0.7Sr03M0.5'M0.5''O3 were grown with total film thickness between 25-50 nm.
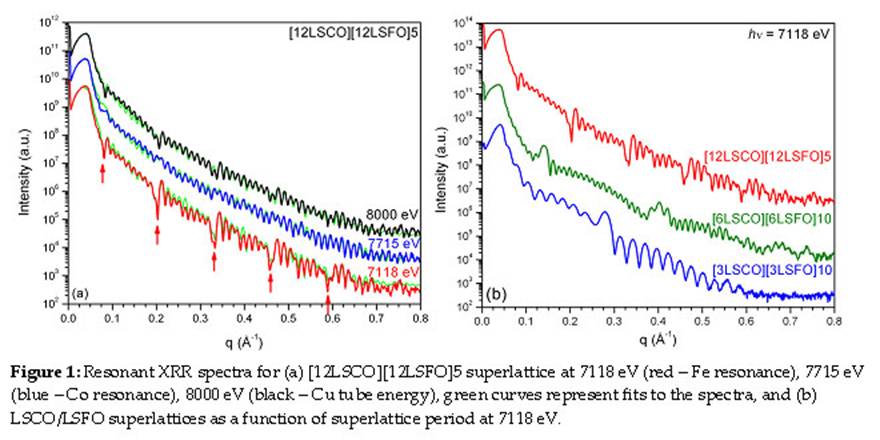
X-ray diffraction (XRD) and x-ray absorption (XA) spectroscopy measurements were performed to determine the structural and chemical properties, respectively, of the epitaxial thin films and superlattices. XRD measurements were carried out on a Bruker D8 Discover four-circle diffractometer located in Takamura's lab, and beamlines 2-1 and 7-2 at the Stanford Synchrotron Radiation Laboratory (SSRL). Capabilities include x-ray reflectometry (XRR), high-resolution XRD, reciprocal space mapping, and grazing incidence diffraction to obtain information about out-of-plane and in-plane lattice parameters, film/substrate crystallographic relationships, film thickness, density, and interface roughness. These measurements confirm that the films and superlattices are epitaxial, fully strained to the underlying substrate, and possess smooth interfaces and the desired thickness/periodicity. Resonant XRR was performed at SSRL by tuning the x-ray energy to the absorption edge of one of the component elements to enhance the contrast between the perovskite layers with similar densities. Fig. 1 plots the resonant XRR spectra for a [12LSCO][12LSFO]5 superlattice at 7118 eV (Fe resonance), 7715 eV (Co resonance), and 8000 eV (Cu tube energy), and for three LSCO/LSFO superlattices at 7118 eV. The persistence of the oscillations to large q values attests to the smoothness of the interfaces. The period of the fast oscillations provides the total thickness of the superlattice, and the locations of superlattice peaks/valleys provide the superlattice period.
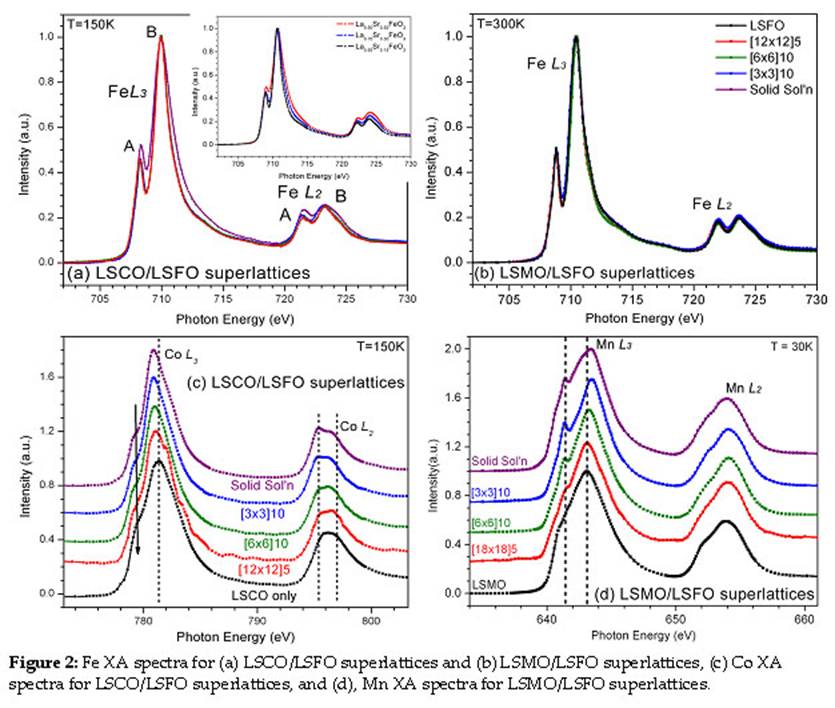
The XA spectra of the Fe, Co, and Mn L3 and L2 absorption edges for the LSCO/LSFO and LSMO/LSFO superlattices and associated single layer and solid solution films are plotted in Fig. 2. Subtle changes in the spectral shape are caused by changes in the valence state of the transition metal ions. For the LSCO/LSFO superlattices, the Fe spectra remain the same regardless of the period, while the solid solution has enhanced intensity of the A' peak and increased width of the B' peak, indicative of decreased Fe3+ concentration. In contrast, the LSMO/LSFO system displays the spectra for increased Fe3+ concentration relative to the LSCO/LSFO series. The corresponding Mn spectra display the appearance of two characteristics as the period decreases: a shift of the main L3 peak to higher energies and the appearance of a second peak at 2eV below the main peak. These features are ascribed to increased Mn4+ contribution. For the Co spectra, the main L3 peak shifts to lower energies with decreasing period, suggesting increased Co3+ concentration. These changes are observed despite a uniform La3+/Sr2+ doping level throughout the superlattices. These results suggest an electronic reconstruction occurs at the interfaces such that an electron transfers from LSMO layers to LSFO layers (Mn3+ → Mn4+) across the interface as proposed by Kumigashira et al, and from LSFO layers to LSCO layers (Fe3+ → Fe4+ and Co4+ → Co3+). These interfacial phenomena directly affect the functional properties of the superlattices in a manner independent from chemical or strain effects.
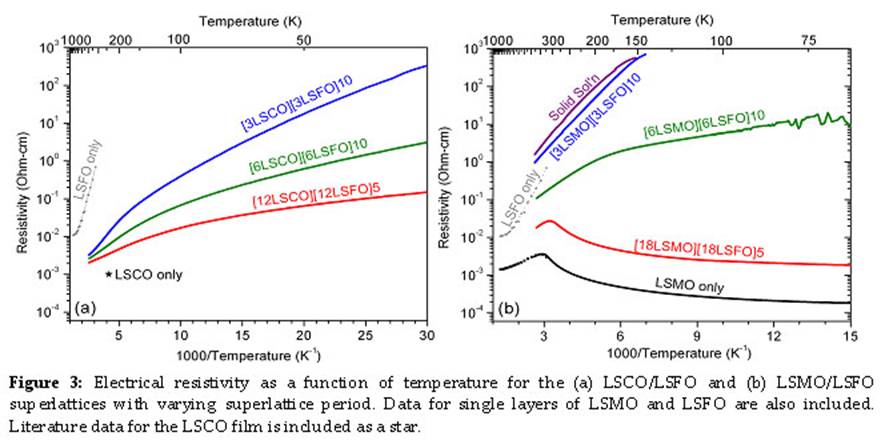
The low temperature electrical resistivity of the LSCO/LSFO and LSMO/LSFO superlattices was measured using the van der Pauw geometry. High temperature AC impedance and DC measurements of the overall resistivity were performed using a two point probe method. All LSFO/LSCO superlattices (Fig. 3) displayed insulating characteristics across the temperature range investigated with a gradual change in the slope between 100-200K with larger activation energy at higher temperatures. The resistivity of these chemically equivalent superlattices at 50K differs by nearly three orders of magnitude, while at room temperature the difference is only slightly greater than a factor of two. The resistivity trends from the LSCO film towards the LSFO film with decreasing period. For the LSMO/LSFO superlattices, we observe a trend of increasing resistivity with decreasing period with two orders of magnitude difference in resistivity at room temperature. Furthermore, the electrical characteristics change with period, such that the LSMO film and [18LSMO][18LSFO]5 superlattice display a metal/insulator transition around room temperature; and the solid solution and [3LSMO][3LSFO]10 superlattice display purely insulating characteristics. The intermediate [6LSMO][6LSFO]10 superlattice displays insulating characteristics and changes slope between 150-200K. These results suggest the resistivity of superlattices can be tuned through the superlattice period by harnessing surface and interfacial phenomena such as electronic reconstruction. Further high temperature resistivity measurements will be performed on the superlattice structures.